Multi-Antigen Outer Membrane Vesicle Engineering to Develop Polyvalent Vaccines: The Staphylococcus aureus Case
- PMID: 34819933
- PMCID: PMC8606680
- DOI: 10.3389/fimmu.2021.752168
Multi-Antigen Outer Membrane Vesicle Engineering to Develop Polyvalent Vaccines: The Staphylococcus aureus Case
Abstract
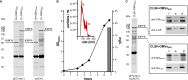
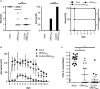
Modification of surface antigens and differential expression of virulence factors are frequent strategies pathogens adopt to escape the host immune system. These escape mechanisms make pathogens a "moving target" for our immune system and represent a challenge for the development of vaccines, which require more than one antigen to be efficacious. Therefore, the availability of strategies, which simplify vaccine design, is highly desirable. Bacterial Outer Membrane Vesicles (OMVs) are a promising vaccine platform for their built-in adjuvanticity, ease of purification and flexibility to be engineered with foreign proteins. However, data on if and how OMVs can be engineered with multiple antigens is limited. In this work, we report a multi-antigen expression strategy based on the co-expression of two chimeras, each constituted by head-to-tail fusions of immunogenic proteins, in the same OMV-producing strain. We tested the strategy to develop a vaccine against Staphylococcus aureus, a Gram-positive human pathogen responsible for a large number of community and hospital-acquired diseases. Here we describe an OMV-based vaccine in which four S. aureus virulent factors, ClfAY338A, LukE, SpAKKAA and HlaH35L have been co-expressed in the same OMVs (CLSH-OMVsΔ60). The vaccine elicited antigen-specific antibodies with functional activity, as judged by their capacity to promote opsonophagocytosis and to inhibit Hla-mediated hemolysis, LukED-mediated leukocyte killing, and ClfA-mediated S. aureus binding to fibrinogen. Mice vaccinated with CLSH-OMVsΔ60 were robustly protected from S. aureus challenge in the skin, sepsis and kidney abscess models. This study not only describes a generalized approach to develop easy-to-produce and inexpensive multi-component vaccines, but also proposes a new tetravalent vaccine candidate ready to move to development.
Keywords: OMV engineering; Staphylococcus aureus; chimeric proteins; multivalent vaccines; outer membrane vesicles (OMVs).
Copyright © 2021 König, Gagliardi, Riedmiller, Andretta, Tomasi, Irene, Frattini, Zanella, Berti, Grandi, Caproni, Fantappiè and Grandi.
Conflict of interest statement
Author FB was employed by company GlaxoSmithKline Vaccines. GG, EK, IZ, CI, and LFa are coinventors of a patent on OMVs. AGr and GG are involved in a biotech company interested in exploiting the OMV platform. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Plotkin SA, Orenstein WA, Offit PA, Edwards KM. eds. Vaccines. 7th ed. Philadelphia: Elsevier; (2018).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

