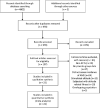
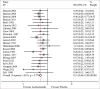
Efficacy of acetazolamide for the prophylaxis of acute mountain sickness: A systematic review, meta-analysis, and trial sequential analysis of randomized clinical trials
- PMID: 34820021
- PMCID: PMC8588948
- DOI: 10.4103/atm.atm_651_20
Efficacy of acetazolamide for the prophylaxis of acute mountain sickness: A systematic review, meta-analysis, and trial sequential analysis of randomized clinical trials
Abstract
Background: Acute mountain sickness (AMS) is a benign and self-limiting syndrome, but can progress to life-threatening conditions if leave untreated. This study aimed to assess the efficacy of acetazolamide for the prophylaxis of AMS, and disclose factors that affect the treatment effect of acetazolamide.
Methods: Randomized controlled trials comparing the use of acetazolamide versus placebo for the prevention of AMS were included. The incidence of AMS was our primary endpoint. Meta-regression analysis was conducted to explore factors that associated with acetazolamide efficacy. Trial sequential analyses were conducted to estimate the statistical power of the available data.
Results: A total of 22 trials were included. Acetazolamide at 125, 250, and 375 mg/bid significantly reduced incidence of AMS compared to placebo. TAS indicated that the current evidence was adequate confirming the efficacy of acetazolamide at 125, 250, and 375 mg/bid in lowering incidence of AMS. There was no evidence of an association between efficacy and dose of acetazolamide, timing at start of acetazolamide treatment, mode of ascent, AMS assessment score, timing of AMS assessment, baseline altitude, and endpoint altitude.
Conclusion: Acetazolamide is effective prophylaxis for the prevention of AMS at 125, 250, and 375 mg/bid. Future investigation should focus on personal characteristics, disclosing the correlation between acetazolamide efficacy and body mass, height, degree of prior acclimatization, individual inborn susceptibility, and history of AMS.
Keywords: Acetazolamide; acute mountain sickness; high altitude; prophylaxis; randomized controlled trials.
Copyright: © 2021 Annals of Thoracic Medicine.
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
Efficacy of Acetazolamide for the Prophylaxis of Acute Mountain Sickness: A Systematic Review, Meta-Analysis and Trial Sequential Analysis of Randomized Clinical Trials.Am J Med Sci. 2021 May;361(5):635-645. doi: 10.1016/j.amjms.2020.12.022. Epub 2021 Feb 12. Am J Med Sci. 2021. PMID: 33587912
-
Efficacy of low-dose acetazolamide (125 mg BID) for the prophylaxis of acute mountain sickness: a prospective, double-blind, randomized, placebo-controlled trial.High Alt Med Biol. 2003 Spring;4(1):45-52. doi: 10.1089/152702903321488979. High Alt Med Biol. 2003. PMID: 12713711 Clinical Trial.
-
Effects of acetazolamide on pulmonary artery pressure and prevention of high-altitude pulmonary edema after rapid active ascent to 4,559 m.J Appl Physiol (1985). 2022 Jun 1;132(6):1361-1369. doi: 10.1152/japplphysiol.00806.2021. Epub 2022 May 5. J Appl Physiol (1985). 2022. PMID: 35511718 Clinical Trial.
-
Altitude, Acute Mountain Sickness, and Acetazolamide: Recommendations for Rapid Ascent.High Alt Med Biol. 2021 Mar;22(1):5-13. doi: 10.1089/ham.2019.0123. Epub 2020 Sep 23. High Alt Med Biol. 2021. PMID: 32975448
-
Flying to high-altitude destinations: Is the risk of acute mountain sickness greater?J Travel Med. 2023 Jun 23;30(4):taad011. doi: 10.1093/jtm/taad011. J Travel Med. 2023. PMID: 36694981 Free PMC article. Review.
Cited by
-
The combined use of acetazolamide and Rhodiola in the prevention and treatment of altitude sickness.Ann Transl Med. 2022 May;10(10):541. doi: 10.21037/atm-22-2111. Ann Transl Med. 2022. PMID: 35722398 Free PMC article.
-
Progress in the Treatment of High Altitude Cerebral Edema: Targeting REDOX Homeostasis.J Inflamm Res. 2023 Jun 23;16:2645-2660. doi: 10.2147/JIR.S415695. eCollection 2023. J Inflamm Res. 2023. PMID: 37383357 Free PMC article. Review.
-
Variables Influencing the Pressure and Volume of the Pulmonary Circulation as Risk Factors for Developing High Altitude Pulmonary Edema (HAPE).Int J Environ Res Public Health. 2022 Oct 26;19(21):13887. doi: 10.3390/ijerph192113887. Int J Environ Res Public Health. 2022. PMID: 36360767 Free PMC article.
-
Prochlorperazine maleate versus placebo for the prevention of acute mountain sickness: study protocol for a randomized controlled trial.Trials. 2024 Nov 21;25(1):785. doi: 10.1186/s13063-024-08592-x. Trials. 2024. PMID: 39574186 Free PMC article.
-
Safety and efficacy of electro-thumbtack needle for acute mountain sickness patients: a protocol of a randomized, single-blinded, and placebo-controlled study.BMC Complement Med Ther. 2024 Oct 3;24(1):355. doi: 10.1186/s12906-024-04655-3. BMC Complement Med Ther. 2024. PMID: 39363177 Free PMC article.
References
-
- Hartman-Ksycińska A, Kluz-Zawadzka J, Lewandowski B. High altitude illness Przegl Epidemiol. 2016;70:490–9. - PubMed
-
- Vardy J, Vardy J, Judge K. Acute mountain sickness and ascent rates in trekkers above 2500 m in the Nepali Himalaya. Aviat Space Environ Med. 2006;77:742–4. - PubMed
-
- Karinen H, Peltonen J, Tikkanen H. Prevalence of acute mountain sickness among Finnish trekkers on Mount Kilimanjaro, Tanzania: An observational study. High Alt Med Biol. 2008;9:301–6. - PubMed
-
- Jackson SJ, Varley J, Sellers C, Josephs K, Codrington L, Duke G, et al. Incidence and predictors of acute mountain sickness among trekkers on Mount Kilimanjaro. High Alt Med Biol. 2010;11:217–22. - PubMed
-
- Beidleman BA, Fulco CS, Muza SR, Rock PB, Staab JE, Forte VA, et al. Effect of six days of staging on physiologic adjustments and acute mountain sickness during ascent to 4300 meters. High Alt Med Biol. 2009;10:253–60. - PubMed
LinkOut - more resources
Full Text Sources