Gluing Living Bone Using a Biomimetic Bioadhesive: From Initial Cut to Final Healing
- PMID: 34820360
- PMCID: PMC8606677
- DOI: 10.3389/fbioe.2021.728042
Gluing Living Bone Using a Biomimetic Bioadhesive: From Initial Cut to Final Healing
Abstract
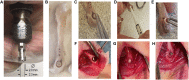
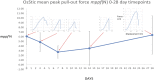
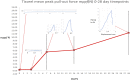
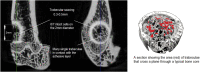
Osteoporotic fractures are a growing issue due to the increasing incidence of osteoporosis worldwide. High reoperation rates in osteoporotic fractures call for investigation into new methods in improving fixation of osteoporotic bones. In the present study, the strength of a recently developed bone bioadhesive, OsStictm, was evaluated in vivo using a novel bone core assay in a murine animal model at 0, 3, 7, 14, 28, and 42 days. Histology and micro-CT were obtained at all time points, and the mean peak pull-out force was assessed on days 0-28. The adhesive provided immediate fixation to the bone core. The mean peak bone core pull-out force gradually decreased from 6.09 N (σ 1.77 N) at day 0 to a minimum of 3.09 N (σ 1.08 N) at day 7, recovering to 6.37 N (σ 4.18 N) by day 28. The corresponding fibrin (Tisseel) control mean peak bone core pull-out characteristic was 0.27 N (σ 0.27 N) at day 0, with an abrupt increase from 0.37 N (σ 0.28) at day 3, 6.39 N (σ 5.09 N) at day 7, and continuing to increase to 11.34 N (σ 6.5 N) by day 28. The bone cores failed either through core pull-out or by the cancellous part of the core fracturing. Overall, the adhesive does not interrupt healing with pathological changes or rapid resorption. Initially, the adhesive bonded the bone core to the femur, and over time, the adhesive was replaced by a vascularised bone of equivalent quality and quantity to the original bone. At the 42 day time point, 70% of the adhesive in the cancellous compartment and 50% in the cortical compartment had been replaced. The adhesive outwith the bone shell was metabolized by cells that are only removing the material excess with no ectopic bone formation. It is concluded that the adhesive is not a physical and biochemical barrier as the bone heals through the adhesive and is replaced by a normal bone tissue. This adhesive composition meets many of the clinical unmet needs expressed in the literature, and may, after further preclinical assessments, have potential in the repair of bone and osteochondral fragments.
Keywords: biomechanical model; biomimetic; bone adhesive; calcium phosphate cement (CPC); fracture healing; orthobiologic; phosphoserine.
Copyright © 2021 Procter, Hulsart-Billström, Alves, Pujari-Palmer, Wenner, Insley, Engqvist and Larsson.
Conflict of interest statement
MP-P, GI, PP, HE authors declare partial ownership in a company that owns all related intellectual property (Biomimetic Innovations Ltd). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









Similar articles
-
A new bone adhesive candidate- does it work in human bone? An ex-vivo preclinical evaluation in fresh human osteoporotic femoral head bone.Injury. 2022 Jun;53(6):1858-1866. doi: 10.1016/j.injury.2022.04.007. Epub 2022 Apr 15. Injury. 2022. PMID: 35469636
-
A biomechanical test model for evaluating osseous and osteochondral tissue adhesives.BMC Biomed Eng. 2019 May 7;1:11. doi: 10.1186/s42490-019-0011-2. eCollection 2019. BMC Biomed Eng. 2019. PMID: 32903290 Free PMC article.
-
Influence of cement compressive strength and porosity on augmentation performance in a model of orthopedic screw pull-out.J Mech Behav Biomed Mater. 2018 Jan;77:624-633. doi: 10.1016/j.jmbbm.2017.10.016. Epub 2017 Oct 13. J Mech Behav Biomed Mater. 2018. PMID: 29100205
-
Combined Percutaneous Iliosacral Screw Fixation With Sacroplasty Using Resorbable Calcium Phosphate Cement for Osteoporotic Pelvic Fractures Requiring Surgery.J Orthop Trauma. 2016 Jun;30(6):e217-22. doi: 10.1097/BOT.0000000000000520. J Orthop Trauma. 2016. PMID: 26741641 Review.
-
Biomechanical and biological aspects of defect treatment in fractures using helical plates.Acta Chir Orthop Traumatol Cech. 2014;81(4):267-71. Acta Chir Orthop Traumatol Cech. 2014. PMID: 25137496 Review.
Cited by
-
In Vitro and In Vivo Evaluation of a Bio-Inspired Adhesive for Bone Fixation.Pharmaceutics. 2023 Apr 13;15(4):1233. doi: 10.3390/pharmaceutics15041233. Pharmaceutics. 2023. PMID: 37111718 Free PMC article.
-
Cytocompatibility and Bioactive Ion Release Profiles of Phosphoserine Bone Adhesive: Bridge from In Vitro to In Vivo.Biomedicines. 2022 Mar 22;10(4):736. doi: 10.3390/biomedicines10040736. Biomedicines. 2022. PMID: 35453486 Free PMC article.
-
Canine ex vivo tarsal arthrodesis: fixation by using a new bone tissue glue.Front Vet Sci. 2023 Sep 20;10:1250147. doi: 10.3389/fvets.2023.1250147. eCollection 2023. Front Vet Sci. 2023. PMID: 37799403 Free PMC article.
References
-
- Adams G. J., Cook R. B., Hutchinson J. R., Zioupos P. (2018). Bone Apparent and Material Densities Examined by Cone Beam Computed Tomography and the Archimedes Technique: Comparison of the Two Methods and Their Results. Front. Mech. Eng. 10.3389/fmech.2017.00023 - DOI
-
- Bystrom J. L., Pujari-Palmer M. (2019). Phosphoserine Functionalized Cements Preserve Metastable Phases, and Reprecipitate Octacalcium Phosphate, Hydroxyapatite, Dicalcium Phosphate, and Amorphous Calcium Phosphate, during Degradation, In Vitro . J. Funct. Biomater. 10 (4), 54. 10.3390/jfb10040054 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

