Vitamin D-Mediated Anti-cancer Activity Involves Iron Homeostatic Balance Disruption and Oxidative Stress Induction in Breast Cancer
- PMID: 34820382
- PMCID: PMC8606886
- DOI: 10.3389/fcell.2021.766978
Vitamin D-Mediated Anti-cancer Activity Involves Iron Homeostatic Balance Disruption and Oxidative Stress Induction in Breast Cancer
Abstract
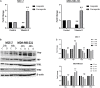
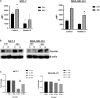
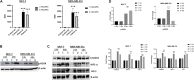
Background: Vitamin D deficiency associates with high risk of breast cancer (BRCA) and increased cellular iron. Vitamin D exerts some of its anti-cancer effects by regulating the expression of key iron regulatory genes (IRGs). The association between vitamin D and cellular iron content in BRCA remains ambiguous. Herein, we addressed whether vitamin D signaling exerts a role in cellular iron homeostasis thereby affecting survival of breast cancer cells. Methods: Expression profile of IRGs in vitamin D-treated breast cancer cells was analyzed using publicly available transcriptomic datasets. After treatment of BRCA cell lines MCF-7 and MDA-MB-231 with the active form of vitamin D, labile iron content, IRGs protein levels, oxidative stress, and cell survival were evaluated. Results: Bioinformatics analysis revealed several IRGs as well as cellular stress relates genes were differentially expressed in BRCA cells. Vitamin D treatment resulted in cellular iron depletion and differentially affected the expression of key IRGs protein levels. Vitamin D treatment exerted oxidative stress induction and alteration in the cellular redox balance by increasing the synthesis of key stress-related markers. Collectively, these effects resulted in a significant decrease in BRCA cell survival. Conclusion: These findings suggest that vitamin D disrupts cellular iron homeostasis leading to oxidative stress induction and cell death.
Keywords: breast cancer; cell death; iron; oxidative stress; vitamin D.
Copyright © 2021 Bajbouj, Sahnoon, Shafarin, Al-Ali, Muhammad, Karim, Guraya and Hamad.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

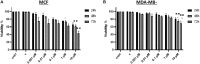



References
-
- Artaza J. N., Sirad F., Ferrini M. G., Norris K. C. (2010). 1,25(OH)2vitamin D3 inhibits cell proliferation by promoting cell cycle arrest without inducing apoptosis and modifies cell morphology of mesenchymal multipotent cells. J. Steroid Biochem. Mol. Biol. 119 73–83. 10.1016/j.jsbmb.2010.01.001 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

