Assessing a method and reference material for quantification of vitamin D binding protein during pregnancy
- PMID: 34820515
- PMCID: PMC8600997
- DOI: 10.1016/j.clinms.2020.01.002
Assessing a method and reference material for quantification of vitamin D binding protein during pregnancy
Abstract
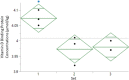
Vitamin D plays a vital role in successful pregnancy outcomes for both the mother and fetus. Vitamin D is bound to vitamin D binding protein (VDBP) in blood and is carried to the liver, kidneys and other target tissues. Accurate measurements of the clinically measured metabolite of vitamin D, 25-hydroxyvitamin D [25(OH)D], depend on complete removal from the binding protein. It has been found that VDBP concentrations increase in maternal serum during pregnancy, obfuscating the accuracy of 25(OH)D concentration measurements in pregnant women. Additionally, measurements of VDBP concentrations during pregnancy have been performed using immunoassays, which suffer from variations due to differences in antibody epitopes, making clinical comparisons difficult. Quantification of VDBP is also of interest because changes in VDBP expression levels may indicate negative outcomes during pregnancy, such as preterm delivery and restricted fetal growth. To address the need for accurate measurement of VDBP during pregnancy, a method using liquid chromatography-isotope dilution mass spectrometry (LC-IDMS) was developed to quantify VDBP using isotopically labeled peptides as internal standards. This method was used to quantify VDBP in Standard Reference Material® (SRM) 1949 Frozen Human Prenatal Serum, which was prepared from separate serum pools of women who were not pregnant and women during each trimester of pregnancy. VDBP concentrations were found to be lowest in the serum pool from non-pregnant women and increased in each trimester. These data had good repeatability and were found to be suitable for reference value assignment of VDBP in SRM 1949.
Keywords: 25(OH)D, 25-hydroxyvitamin D; AAA, amino acid analysis; CV, coefficient of variation; GC-globulin; Isotope-dilution; LC-IDMS, liquid chromatography-isotope dilution mass spectrometry; LC-MS/MS; LC-MS/MS, liquid-chromatography tandem mass spectrometry; MRM; MRM, multiple reaction monitoring; NIST, National Institute of Standards and Technology; PARs, peak area ratios; Pregnancy; Quantification; SRM, Standard Reference Material®; TCEP, tris(2-carboxyethyl)phosphine hydrochloride; TFA, trifluoroacetic acid; TFE, trifluoroethanol; VDBP, vitamin D binding protein; Vitamin D binding protein.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Sempos C.T., Vesper H.W., Phinney K.W., Thienpont L.M., Coates P.M. Vitamin D status as an international issue: national surveys and the problem of standardization. Scand. J. Clin. Lab. Invest. Suppl. 2012;243:32–40. - PubMed
-
- Sempos C.T., Heijboer A.C., Bikle D.D., Bollerslev J., Bouillon R., Brannon P.M., DeLuca H.F., Jones G., Munns C.F., Bilezikian J.P., Giustina A., Binkley N. Vitamin D assays and the definition of hypovitaminosis D: results from the First International Conference on Controversies in Vitamin D. Br. J. Clin. Pharmacol. 2018;84:2194–2207. - PMC - PubMed
-
- Hilger J., Friedel A., Herr R., Rausch T., Roos F., Wahl D.A., Pierroz D.D., Weber P., Hoffmann K. A systematic review of vitamin D status in populations worldwide. Br. J. Nutr. 2014;111:23–45. - PubMed
-
- Holick M.F. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017;18:153–165. - PubMed
-
- Cashman K.D., Dowling K.G., Skrabakova Z., Gonzalez-Gross M., Valtuena J., De Henauw S., Moreno L., Damsgaard C.T., Michaelsen K.F., Molgaard C., Jorde R., Grimnes G., Moschonis G., Mavrogianni C., Manios Y., Thamm M., Mensink G.B., Rabenberg M., Busch M.A., Cox L., Meadows S., Goldberg G., Prentice A., Dekker J.M., Nijpels G., Pilz S., Swart K.M., van Schoor N.M., Lips P., Eiriksdottir G., Gudnason V., Cotch M.F., Koskinen S., Lamberg-Allardt C., Durazo-Arvizu R.A., Sempos C.T., Kiely M. Vitamin D deficiency in Europe: pandemic? Am. J. Clin. Nutr. 2016;103:1033–1044. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
