The Data Use Ontology to streamline responsible access to human biomedical datasets
- PMID: 34820659
- PMCID: PMC8591903
- DOI: 10.1016/j.xgen.2021.100028
The Data Use Ontology to streamline responsible access to human biomedical datasets
Abstract
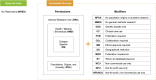
Human biomedical datasets that are critical for research and clinical studies to benefit human health also often contain sensitive or potentially identifying information of individual participants. Thus, care must be taken when they are processed and made available to comply with ethical and regulatory frameworks and informed consent data conditions. To enable and streamline data access for these biomedical datasets, the Global Alliance for Genomics and Health (GA4GH) Data Use and Researcher Identities (DURI) work stream developed and approved the Data Use Ontology (DUO) standard. DUO is a hierarchical vocabulary of human and machine-readable data use terms that consistently and unambiguously represents a dataset's allowable data uses. DUO has been implemented by major international stakeholders such as the Broad and Sanger Institutes and is currently used in annotation of over 200,000 datasets worldwide. Using DUO in data management and access facilitates researchers' discovery and access of relevant datasets. DUO annotations increase the FAIRness of datasets and support data linkages using common data use profiles when integrating the data for secondary analyses. DUO is implemented in the Web Ontology Language (OWL) and, to increase community awareness and engagement, hosted in an open, centralized GitHub repository. DUO, together with the GA4GH Passport standard, offers a new, efficient, and streamlined data authorization and access framework that has enabled increased sharing of biomedical datasets worldwide.
Keywords: FAIR; GA4GH; automated data access; consent; controlled access; data access; data restrictions; ontology; secondary data use; standard.
© 2021 The Author(s).
Conflict of interest statement
M.N.C. is an employee of Foundation Medicine and equity holder of Roche. A.A.P. is a venture partner at GV and an employee of alphabet corporation. He has received funding from MSFT, Verily, IBM, Intel, Bayer, and Novartis. The views expressed by L.L.R. are the author’s own and do not necessarily represent those of her organization.
Figures



References
-
- GA4GH Data Use and Researcher ID work stream https://ga4gh-duri.github.io/
Grants and funding
LinkOut - more resources
Full Text Sources

