Treatment and outcomes of dogs with hepatocutaneous syndrome or hepatocutaneous-associated hepatopathy
- PMID: 34820906
- PMCID: PMC8783367
- DOI: 10.1111/jvim.16323
Treatment and outcomes of dogs with hepatocutaneous syndrome or hepatocutaneous-associated hepatopathy
Abstract
Background: Superficial necrolytic dermatitis (SND) in dogs is a rare disorder most commonly associated with hepatocutaneous syndrome. Although often reported as fatal, sporadically reported long-term remissions might be more common than previously believed and linked to treatment regimens.
Hypothesis/objectives: Evaluate treatments and associated outcomes in dogs with hepatocutaneous-associated hepatopathy (HCH) with or without SND, designated collectively aminoaciduric canine hypoaminoacidemic hepatopathy syndrome (ACHES).
Animals: Forty-one dogs of various breeds and ages diagnosed with ACHES.
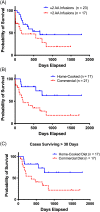
Methods: Retrospective study. Electronic surveys, medical records (2014-2019), and communication with veterinarians provided data. Three treatment categories were each dichotomized: IV amino acid (IV-AA) infusions (≥2 vs <2), supplements including S-adenosylmethionine (SAMe), arginine with ornithine, glutathione, lysine, proline, omega-3 fatty acids, or zinc (≥3 vs <3), and diet type (home-cooked vs commercial). Optimal treatment was defined as receiving ≥2 IV-AA treatments, ≥3 nutritional supplements, and a home-cooked diet.
Results: Most dogs (29/41, 71%) received IV-AA infusions (23/29, ≥2 infusions). Twenty-one dogs (51%) were fed commercial diets; 17/41 (41%) were fed home-cooked diets. Most dogs received SAMe (32/41, 78%) and a median of 3 supplements. In 4 dogs, HCH remission occurred. Overall all-cause median survival time (MST) was 359 days, and disease-specific MST was 557 days (range, 1-1783 days). Optimally treated dogs (n = 9) lived significantly longer (MST, >1783 days, P = .02) than variably treated dogs (MST, 214 days).
Conclusions and clinical importance: Optimized ACHES management can resolve SND and HCH and confer long-term survival.
Keywords: hepatic disease; internal medicine-canine; metabolic disease.
© 2021 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals LLC on behalf of American College of Veterinary Internal Medicine.
Conflict of interest statement
Authors declare no conflict of interest.
Figures





References
-
- Becker SW, Kahn D, Rothman S. Cutaneous manifestations of internal malignant tumors. Arch Dermatol. 1942;45:1069. - PubMed
-
- Walton DK, Center SA, Scott DW, et al. Ulcerative dermatosis associated with diabetes‐mellitus in the dog—a report of 4 cases. J Am Anim Hosp Assoc. 1986;22:79‐88.
-
- Hall‐Fonte DL, Center SA, McDonough SP, et al. Hepatocutaneous syndrome in Shih Tzus: 31 cases (1996‐2014). J Am Vet Med Assoc. 2016;248:802‐813. - PubMed
-
- Byrne KP. Metabolic epidermal necrosis‐hepatocutaneous syndrome. Vet Clin North Am Anim Pract. 1999;29:1337‐1355. - PubMed
-
- Loftus JP, Center SA, Lucy JM, et al. Characterization of aminoaciduria and hypoaminoacidemia in dogs with hepatocutaneous syndrome. Am J Vet Res. 2017;78:735‐744. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
