Comparison of the immunogenicity of BNT162b2 and CoronaVac COVID-19 vaccines in Hong Kong
- PMID: 34820940
- PMCID: PMC8934254
- DOI: 10.1111/resp.14191
Comparison of the immunogenicity of BNT162b2 and CoronaVac COVID-19 vaccines in Hong Kong
Abstract
Background and objective: Few head-to-head evaluations of immune responses to different vaccines have been reported.
Methods: Surrogate virus neutralization test (sVNT) antibody levels of adults receiving either two doses of BNT162b2 (n = 366) or CoronaVac (n = 360) vaccines in Hong Kong were determined. An age-matched subgroup (BNT162b2 [n = 49] vs. CoronaVac [n = 49]) was tested for plaque reduction neutralization (PRNT) and spike-binding antibody and T-cell reactivity in peripheral blood mononuclear cells.
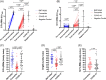
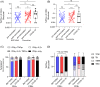
Results: One month after the second dose of vaccine, BNT162b2 elicited significantly higher PRNT50 , PRNT90 , sVNT, spike receptor binding, spike N-terminal domain binding, spike S2 domain binding, spike FcR binding and antibody avidity levels than CoronaVac. The geometric mean PRNT50 titres in those vaccinated with BNT162b2 and CoronaVac vaccines were 251.6 and 69.45, while PRNT90 titres were 98.91 and 16.57, respectively. All of those vaccinated with BNT162b2 and 45 (91.8%) of 49 vaccinated with CoronaVac achieved the 50% protection threshold for PRNT90. Allowing for an expected seven-fold waning of antibody titres over 6 months for those receiving CoronaVac, only 16.3% would meet the 50% protection threshold versus 79.6% of BNT162b2 vaccinees. Age was negatively correlated with PRNT90 antibody titres. Both vaccines induced SARS-CoV-2-specific CD4+ and CD8+ T-cell responses at 1 month post-vaccination but CoronaVac elicited significantly higher structural protein-specific CD4+ and CD8+ T-cell responses.
Conclusion: Vaccination with BNT162b2 induces stronger humoral responses than CoronaVac. CoronaVac induces higher CD4+ and CD8+ T-cell responses to the structural protein than BNT162b2.
Keywords: BNT162b2; Biontech; COVID-19; CoronaVac; SARS-CoV-2; Sinovac; coronavirus disease; immunogenicity.
© 2021 The Authors. Respirology published by John Wiley & Sons Australia, Ltd on behalf of Asian Pacific Society of Respirology.
Conflict of interest statement
The study was partly supported by Fast Grant ##2161 (Emergent Ventures to Gaya K. Amerasinghe) and NIH grants (P01AI120943 and R01AI123926 to Gaya K. Amerasinghe; R01AI107056 to Daisy W. Leung).
Figures

Comment in
-
COVID-19 vaccine-induced immunity: Head-to-head comparison of mRNA (BNT162b2) versus inactivated (CoronaVac) vaccines.Respirology. 2022 Apr;27(4):260-261. doi: 10.1111/resp.14236. Epub 2022 Feb 28. Respirology. 2022. PMID: 35229416 Free PMC article.
References
-
- World Health Organization WHO website. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Access on 25/10/2021.
-
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nat Med. 2021;27(7):1205–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2020A0505100063/Guangdong Province International Scientific and Technological Cooperation Projects
- NRF-2018M3A9H4055203/National Research Foundation of Korea (NRF)
- R01 AI107056/AI/NIAID NIH HHS/United States
- 2161/Fast Grant
- HHSN272201400006C/AI/NIAID NIH HHS/United States
- P01AI120943/US National Institutes of Health
- T11-705/21-N/National Natural Science Foundation of China (NSFC)/Research Grants Council (RGC) Joint Research Scheme
- Pasteur Foundation Asia
- P01 AI120943/AI/NIAID NIH HHS/United States
- COVID1903003/Health and Medical Research Fund Commissioned Research on the Novel Coronavirus Disease (COVID-19), Hong Kong SAR
- N_HKU737/18/National Natural Science Foundation of China (NSFC)/Research Grants Council (RGC) Joint Research Scheme
- R01AI123926/US National Institutes of Health
- T11-712/19-N/National Natural Science Foundation of China (NSFC)/Research Grants Council (RGC) Joint Research Scheme
- COVID-190126/Health and Medical Research Fund Commissioned Research on the Novel Coronavirus Disease (COVID-19), Hong Kong SAR
- R01AI107056/US National Institutes of Health
- COVID-190115/Health and Medical Research Fund Commissioned Research on the Novel Coronavirus Disease (COVID-19), Hong Kong SAR
- R01 AI123926/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

