Assessment of Pharmacokinetic Interaction Between Gefapixant (MK-7264), a P2X3 Receptor Antagonist, and the OATP1B1 Drug Transporter Substrate Pitavastatin
- PMID: 34821075
- PMCID: PMC9298894
- DOI: 10.1002/cpdd.1047
Assessment of Pharmacokinetic Interaction Between Gefapixant (MK-7264), a P2X3 Receptor Antagonist, and the OATP1B1 Drug Transporter Substrate Pitavastatin
Abstract
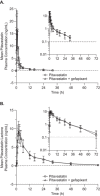
Gefapixant (MK-7264, AF-219), a first-in-class P2X3 antagonist, is being developed as oral treatment for refractory or unexplained chronic cough. Based on in vitro data, gefapixant exerts inhibitory activity on the organic anion transporter (OAT) P1B1 transporter. Therefore, a drug-drug interaction study evaluating the potential effects of gefapixant on the OATP1B1 drug transporter, using pitavastatin as a sensitive probe substrate, was conducted. An open-label, 2-period, fixed-sequence study in 20 healthy adults 18 to 55 years old was conducted. In period 1, a 1-mg oral dose of pitavastatin was administered to each participant. After a ≥4-day washout, in period 2 participants received a 45-mg oral dose of gefapixant twice daily on days 1 through 4. On day 2 of period 2, pitavastatin was coadministered with the morning dose of gefapixant. Pitavastatin exposures following single-dose administration with and without multiple doses of gefapixant were similar: geometric mean ratio (90% confidence interval) of pitavastatin area under the plasma concentration-time curve from time 0 to infinity (AUC0-∞ ) (pitavastatin + gefapixant/pitavastatin alone) was 0.97 (0.93-1.02). The ratio of pitavastatin lactone AUC0-∞ to pitavastatin AUC0-∞ was also comparable between treatments. Administration of gefapixant and pitavastatin was generally well tolerated, with no safety findings of concern. These results support that gefapixant has a low potential to inhibit the OATP1B1 transporter.
Keywords: chronic cough; drug-drug interaction; organic anion transporting polypeptide.
© 2021 Merck Sharp & Dohme Corp. Clinical Pharmacology in Drug Development published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
J.B.M., A.H., B.M., G.C.G., R.E., S.A.S., and M.I. are current or former employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, and may own stock/stock options in Merck & Co., Inc.. J.E.L. declares no conflicts of interest.
Figures
References
-
- Schappert SM, Burt CW. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 2001‐02. Vital Health Stat. 2006;13(159):1‐66. - PubMed
-
- Hsiao CJ, Cherry DK, Beatty PC, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2007 summary. Natl Health Stat Report. 2010(27):1‐32. - PubMed
-
- Haque RA, Usmani OS, Barnes PJ. Chronic idiopathic cough: a discrete clinical entity? Chest. 2005;127(5):1710‐1713. - PubMed
-
- Chung KF, McGarvey L, Mazzone SB. Chronic cough as a neuropathic disorder. Lancet Respir Med. 2013;1(5):414‐422. - PubMed
-
- McCrory DC, Coeytaux RR, Yancy WS Jr, et al. Assessment and Management of Chronic Cough. AHRQ Comparative Effectiveness Reviews. Rockville, MD: US Department of Health & Human Services; 2013. https://effectivehealthcare.ahrq.gov/products/chronic-cough/research. Accessed October 7, 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

