Sepsis induces interleukin 6, gp130/JAK2/STAT3, and muscle wasting
- PMID: 34821076
- PMCID: PMC8818599
- DOI: 10.1002/jcsm.12867
Sepsis induces interleukin 6, gp130/JAK2/STAT3, and muscle wasting
Abstract
Background: Sepsis and inflammation can cause intensive care unit-acquired weakness (ICUAW). Increased interleukin-6 (IL-6) plasma levels are a risk factor for ICUAW. IL-6 signalling involves the glycoprotein 130 (gp130) receptor and the JAK/STAT-pathway, but its role in sepsis-induced muscle wasting is uncertain. In a clinical observational study, we found that the IL-6 target gene, SOCS3, was increased in skeletal muscle of ICUAW patients indicative for JAK/STAT-pathway activation. We tested the hypothesis that the IL-6/gp130-pathway mediates ICUAW muscle atrophy.
Methods: We sequenced RNA (RNAseq) from tibialis anterior (TA) muscle of cecal ligation and puncture-operated (CLP) and sham-operated wildtype (WT) mice. The effects of the IL-6/gp130/JAK2/STAT3-pathway were investigated by analysing the atrophy phenotype, gene expression, and protein contents of C2C12 myotubes. Mice lacking Il6st, encoding gp130, in myocytes (cKO) and WT controls, as well as mice treated with the JAK2 inhibitor AG490 or vehicle were exposed to CLP or sham surgery for 24 or 96 h.
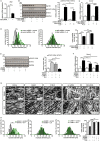
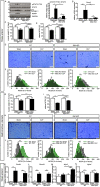
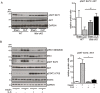
Results: Analyses of differentially expressed genes in RNAseq (≥2-log2-fold change, P < 0.01) revealed an activation of IL-6-signalling and JAK/STAT-signalling pathways in muscle of septic mice, which occurred after 24 h and lasted at least for 96 h during sepsis. IL-6 treatment of C2C12 myotubes induced STAT3 phosphorylation (three-fold, P < 0.01) and Socs3 mRNA expression (3.1-fold, P < 0.01) and caused myotube atrophy. Knockdown of Il6st diminished IL-6-induced STAT3 phosphorylation (-30.0%; P < 0.01), Socs3 mRNA expression, and myotube atrophy. JAK2 (- 29.0%; P < 0.01) or STAT3 inhibition (-38.7%; P < 0.05) decreased IL-6-induced Socs3 mRNA expression. Treatment with either inhibitor attenuated myotube atrophy in response to IL-6. CLP-operated septic mice showed an increased STAT3 phosphorylation and Socs3 mRNA expression in TA muscle, which was reduced in septic Il6st-cKO mice by 67.8% (P < 0.05) and 85.6% (P < 0.001), respectively. CLP caused a loss of TA muscle weight, which was attenuated in Il6st-cKO mice (WT: -22.3%, P < 0.001, cKO: -13.5%, P < 0.001; WT vs. cKO P < 0.001). While loss of Il6st resulted in a reduction of MuRF1 protein contents, Atrogin-1 remained unchanged between septic WT and cKO mice. mRNA expression of Trim63/MuRF1 and Fbxo32/Atrogin-1 were unaltered between CLP-treated WT and cKO mice. AG490 treatment reduced STAT3 phosphorylation (-22.2%, P < 0.05) and attenuated TA muscle atrophy in septic mice (29.6% relative reduction of muscle weight loss, P < 0.05). The reduction in muscle atrophy was accompanied by a reduction in Fbxo32/Atrogin-1-mRNA (-81.3%, P < 0.05) and Trim63/MuRF1-mRNA expression (-77.6%, P < 0.05) and protein content.
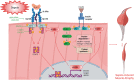
Conclusions: IL-6 via the gp130/JAK2/STAT3-pathway mediates sepsis-induced muscle atrophy possibly contributing to ICUAW.
Keywords: IL-6 signalling; Inflammation; Intensive care unit acquired weakness; Muscle atrophy; Sepsis; gp130.
© 2021 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
Lukas Zanders, Melanie Kny, Alexander Hahn, Sibylle Schmidt, Sebastian Wundersitz, Mihail Todiras, Ines Lahmann, Arnab Bandyopadhyay, Tobias Wollersheim, Lars Kaderali, Friedrich C. Luft, Carmen Birchmeier, Steffen Weber‐Carstens, and Jens Fielitz declare that they have no conflict of interest.
Figures



References
-
- Ali NA, O'Brien JM Jr, Hoffmann SP, Phillips G, Garland A, Finley JC, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med 2008;178:261–268. - PubMed
-
- Sharshar T, Bastuji‐Garin S, Stevens RD, Durand MC, Malissin I, Rodriguez P, et al. Presence and severity of intensive care unit‐acquired paresis at time of awakening are associated with increased intensive care unit and hospital mortality. Crit Care Med 2009;37:3047–3053. - PubMed
-
- Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz‐Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011;364:1293–1304. - PubMed
-
- Wollersheim T, Woehlecke J, Krebs M, Hamati J, Lodka D, Luther‐Schroeder A, et al. Dynamics of myosin degradation in intensive care unit‐acquired weakness during severe critical illness. Intensive Care Med 2014;40:528–538. - PubMed
-
- Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA 2013;310:1591–1600. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

