Stress relaxation in tunable gels
- PMID: 34821243
- PMCID: PMC8612457
- DOI: 10.1039/d1sm00091h
Stress relaxation in tunable gels
Abstract
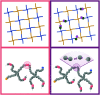
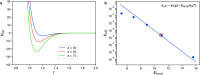
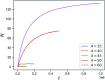
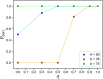
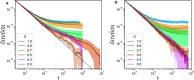
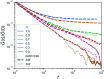
Hydrogels are a staple of biomaterials development. Optimizing their use in e.g. drug delivery or tissue engineering requires a solid understanding of how to adjust their mechanical properties. Here, we present a numerical study of a class of hydrogels made of 4-arm star polymers with a combination of covalent and reversible crosslinks. This design principle combines the flexibility and responsivity associated with reversible linkers with stability provided by chemical crosslinks. In molecular dynamics simulations of such hybrid gel networks, we observe that the strength of the reversible bonds can tune the material from solid to fluid. We identify at what fraction of reversible bonds this tunability is most pronounced, and find that the stress relaxation time of the gels in this tunable regime is set directly by the average lifetime of the reversible bonds. As our design is easy to realize in the already widely-used tetraPEG gel setting, our work will provide guidelines to improve the mechanical performance of biomedical gels.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

