Possibility of exosome‑based coronavirus disease 2019 vaccine (Review)
- PMID: 34821373
- PMCID: PMC8630821
- DOI: 10.3892/mmr.2021.12542
Possibility of exosome‑based coronavirus disease 2019 vaccine (Review)
Abstract
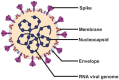
Coronavirus disease 2019 (COVID‑19) is a global pandemic that can have a long‑lasting impact on public health if not properly managed. Ongoing vaccine development trials involve classical molecular strategies based on inactivated or attenuated viruses, single peptides or viral vectors. However, there are multiple issues, such as the risk of reversion to virulence, inability to provide long‑lasting protection and limited protective immunity. To overcome the aforementioned drawbacks of currently available COVID‑19 vaccines, an alternative strategy is required to produce safe and efficacious vaccines that impart long‑term immunity. Exosomes (key intercellular communicators characterized by low immunogenicity, high biocompatibility and innate cargo‑loading capacity) offer a novel approach for effective COVID‑19 vaccine development. An engineered exosome‑based vaccine displaying the four primary structural proteins of SARS‑CoV‑2 (spike, membrane, nucleocapside and envelope proteins) induces humoral and cell mediated immunity and triggers long‑lasting immunity. The present review investigated the prospective use of exosomes in the development of COVID‑19 vaccines; moreover, exosome‑based vaccines may be key to control the COVID‑19 pandemic by providing enhanced protection compared with existing vaccines.
Keywords: coronavirus disease 2019; exosome; pandemic; severe acute respiratory syndrome coronavirus 2; vaccine.
Conflict of interest statement
The purification strategy for the mass production of highly purified and concentrated exosomes is subject to Korean patent application no. 10-2020-0062365, associated with CK-Exogene, Inc. JK and NT are employees of CK-Exogene, Inc. The other authors (KHY, BJK, JOL, YNJ and YJC) are not associated with CK-Exogene, Inc. and declare that they have no competing interests.
Figures


References
-
- Cascella M, Rajnik M, Aleem A, Dulebohn S, Di Napoli R. StatPearls; 2021. Features, Evaluation, and Treatment of Coronavirus (COVID-19) - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

