Assessment of Brain Functional Activity Using a Miniaturized Head-Mounted Scanning Photoacoustic Imaging System in Awake and Freely Moving Rats
- PMID: 34821645
- PMCID: PMC8615926
- DOI: 10.3390/bios11110429
Assessment of Brain Functional Activity Using a Miniaturized Head-Mounted Scanning Photoacoustic Imaging System in Awake and Freely Moving Rats
Abstract
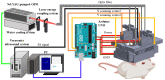
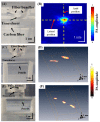
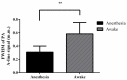
Understanding the relationship between brain function and natural behavior remains a significant challenge in neuroscience because there are very few convincing imaging/recording tools available for the evaluation of awake and freely moving animals. Here, we employed a miniaturized head-mounted scanning photoacoustic imaging (hmPAI) system to image real-time cortical dynamics. A compact photoacoustic (PA) probe based on four in-house optical fiber pads and a single custom-made 48-MHz focused ultrasound transducer was designed to enable focused dark-field PA imaging, and miniature linear motors were included to enable two-dimensional (2D) scanning. The total dimensions and weight of the proposed hmPAI system are only approximately 50 × 64 × 48 mm and 58.7 g (excluding cables). Our ex vivo phantom experimental tests revealed that a spatial resolution of approximately 0.225 mm could be achieved at a depth of 9 mm. Our in vivo results further revealed that the diameters of cortical vessels draining into the superior sagittal sinus (SSS) could be clearly imaged and continuously observed in both anesthetized rats and awake, freely moving rats. Statistical analysis showed that the full width at half maximum (FWHM) of the PA A-line signals (relative to the blood vessel diameter) was significantly increased in the selected SSS-drained cortical vessels of awake rats (0.58 ± 0.17 mm) compared with those of anesthetized rats (0.31 ± 0.09 mm) (p < 0.01, paired t-test). In addition, the number of pixels in PA B-scan images (relative to the cerebral blood volume (CBV)) was also significantly increased in the selected SSS-drained blood vessels of awake rats (107.66 ± 23.02 pixels) compared with those of anesthetized rats (81.99 ± 21.52 pixels) (p < 0.01, paired t-test). This outcome may result from a more active brain in awake rats than in anesthetized rats, which caused cerebral blood vessels to transport more blood to meet the increased nutrient demand of the tissue, resulting in an obvious increase in blood vessel volume. This hmPAI system was further validated for utility in the brains of awake and freely moving rats, showing that their natural behavior was unimpaired during vascular imaging, thereby providing novel opportunities for studies of behavior, cognition, and preclinical models of brain diseases.
Keywords: fiber-bundle-based illumination; freely moving animals; hemoglobin oxygen saturation; in vivo imaging; photoacoustic (PA).
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Revisiting the cerebral hemodynamics of awake, freely moving rats with repeated ketamine self-administration using a miniature photoacoustic imaging system.Neurophotonics. 2022 Oct;9(4):045003. doi: 10.1117/1.NPh.9.4.045003. Epub 2022 Nov 1. Neurophotonics. 2022. PMID: 36338453 Free PMC article.
-
An Adjustable Dark-Field Acoustic-Resolution Photoacoustic Imaging System with Fiber Bundle-Based Illumination.Biosensors (Basel). 2021 Aug 3;11(8):262. doi: 10.3390/bios11080262. Biosensors (Basel). 2021. PMID: 34436064 Free PMC article.
-
Detachable head-mounted photoacoustic microscope in freely moving mice.Opt Lett. 2021 Dec 15;46(24):6055-6058. doi: 10.1364/OL.444226. Opt Lett. 2021. PMID: 34913906
-
Miniature Multiphoton Microscopes for Recording Neural Activity in Freely Moving Animals.2023 Feb 21. In: Papagiakoumou E, editor. All-Optical Methods to Study Neuronal Function [Internet]. New York: Humana; 2023. Chapter 7. 2023 Feb 21. In: Papagiakoumou E, editor. All-Optical Methods to Study Neuronal Function [Internet]. New York: Humana; 2023. Chapter 7. PMID: 38285825 Free Books & Documents. Review.
-
Advances of optical miniscopes for in vivo imaging of neural activity in freely moving animals.Front Neurosci. 2022 Sep 7;16:994079. doi: 10.3389/fnins.2022.994079. eCollection 2022. Front Neurosci. 2022. PMID: 36161177 Free PMC article. Review.
Cited by
-
Revisiting the cerebral hemodynamics of awake, freely moving rats with repeated ketamine self-administration using a miniature photoacoustic imaging system.Neurophotonics. 2022 Oct;9(4):045003. doi: 10.1117/1.NPh.9.4.045003. Epub 2022 Nov 1. Neurophotonics. 2022. PMID: 36338453 Free PMC article.
References
-
- Makeig S., Kothe C., Mullen T., Bigdely-Shamlo N., Zhang Z., Kreutz-Delgado K. Evolving Signal Processing for Brain-Computer Interfaces. Proc. IEEE. 2012;100:1567–1584. doi: 10.1109/JPROC.2012.2185009. - DOI
-
- Lance B.J., Kerick S.E., Ries A.J., Oie K.S., McDowell K. Brain-Computer Interface Technologies in the Coming Decades. Proc. IEEE. 2012;100:1585–1599. doi: 10.1109/JPROC.2012.2184830. - DOI
-
- Garcia-Cortadella R., Schwesig G., Jeschke C., Illa X., Gray A.L., Savage S., Stamatidou E., Schiessl I., Masvidal-Codina E., Kostarelos K., et al. Graphene active sensor arrays for long-term and wireless mapping of wide frequency band epicortical brain activity. Nat. Commun. 2021;12:211. doi: 10.1038/s41467-020-20546-w. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

