Heat-Inactivation of Fetal and Newborn Sera Did Not Impair the Expansion and Scaffold Engineering Potentials of Fibroblasts
- PMID: 34821750
- PMCID: PMC8615100
- DOI: 10.3390/bioengineering8110184
Heat-Inactivation of Fetal and Newborn Sera Did Not Impair the Expansion and Scaffold Engineering Potentials of Fibroblasts
Abstract
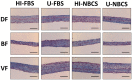
Heat inactivation of bovine sera is routinely performed in cell culture laboratories. Nevertheless, it remains debatable whether it is still necessary due to the improvement of the production process of bovine sera. Do the benefits balance the loss of many proteins, such as hormones and growth factors, that are very useful for cell culture? This is even truer in the case of tissue engineering, the processes of which is often very demanding. This balance is examined here, from nine populations of fibroblasts originating from three different organs, by comparing the capacity of adhesion and proliferation of cells, their metabolism, and the capacity to produce the stroma; their histological appearance, thickness, and mechanical properties were also evaluated. Overall, serum inactivation does not appear to provide a significant benefit.
Keywords: 2D cell culture; heat-inactivation; mechanical properties; metabolism; serum; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Routine heat inactivation of serum reduces its capacity to promote cell attachment.In Vitro Cell Dev Biol. 1987 Oct;23(10):691-7. doi: 10.1007/BF02620982. In Vitro Cell Dev Biol. 1987. PMID: 3667489
-
Analysis of human synovial and bone marrow mesenchymal stem cells in relation to heat-inactivation of autologous and fetal bovine serums.BMC Musculoskelet Disord. 2010 Sep 14;11:208. doi: 10.1186/1471-2474-11-208. BMC Musculoskelet Disord. 2010. PMID: 20840748 Free PMC article.
-
LEUCOCYTIC SECRETIONS.J Exp Med. 1922 Nov 30;36(6):645-59. doi: 10.1084/jem.36.6.645. J Exp Med. 1922. PMID: 19868699 Free PMC article.
-
The Impact of Various Culture Conditions on Human Mesenchymal Stromal Cells Metabolism.Stem Cells Int. 2021 Mar 1;2021:6659244. doi: 10.1155/2021/6659244. eCollection 2021. Stem Cells Int. 2021. PMID: 33727935 Free PMC article.
-
Gamma irradiation of animal sera for inactivation of viruses and mollicutes--a review.Biologicals. 2011 Nov;39(6):370-7. doi: 10.1016/j.biologicals.2011.05.003. Epub 2011 Aug 25. Biologicals. 2011. PMID: 21871817 Review.
Cited by
-
Impact of the Use of 2-Phospho-L Ascorbic Acid in the Production of Engineered Stromal Tissue for Regenerative Medicine.Cells. 2025 Jul 21;14(14):1123. doi: 10.3390/cells14141123. Cells. 2025. PMID: 40710376 Free PMC article.
-
RipE expression correlates with high ATP levels in Ehrlichia, which confers resistance during the extracellular stage to facilitate a new cycle of infection.Front Cell Infect Microbiol. 2024 Oct 1;14:1416577. doi: 10.3389/fcimb.2024.1416577. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39411319 Free PMC article.
-
The dark side of foetal bovine serum in extracellular vesicle studies.J Extracell Vesicles. 2022 Oct;11(10):e12271. doi: 10.1002/jev2.12271. J Extracell Vesicles. 2022. PMID: 36214482 Free PMC article. Review.
-
Tissue Engineering for Penile Reconstruction.Bioengineering (Basel). 2024 Feb 28;11(3):230. doi: 10.3390/bioengineering11030230. Bioengineering (Basel). 2024. PMID: 38534504 Free PMC article. Review.
-
Bisphenols A and S Alter the Bioenergetics and Behaviours of Normal Urothelial and Bladder Cancer Cells.Cancers (Basel). 2022 Aug 19;14(16):4011. doi: 10.3390/cancers14164011. Cancers (Basel). 2022. PMID: 36011004 Free PMC article.
References
-
- Linscott W.D., Triglia R.P. The bovine complement system. Adv. Exp. Med. Biol. 1981;137:413–430. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

