3D Hepatic Organoid-Based Advancements in LIVER Tissue Engineering
- PMID: 34821751
- PMCID: PMC8615121
- DOI: 10.3390/bioengineering8110185
3D Hepatic Organoid-Based Advancements in LIVER Tissue Engineering
Abstract
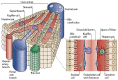
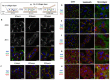
Liver-associated diseases and tissue engineering approaches based on in vitro culture of functional Primary human hepatocytes (PHH) had been restricted by the rapid de-differentiation in 2D culture conditions which restricted their usability. It was proven that cells growing in 3D format can better mimic the in vivo microenvironment, and thus help in maintaining metabolic activity, phenotypic properties, and longevity of the in vitro cultures. Again, the culture method and type of cell population are also recognized as important parameters for functional maintenance of primary hepatocytes. Hepatic organoids formed by self-assembly of hepatic cells are microtissues, and were able to show long-term in vitro maintenance of hepato-specific characteristics. Thus, hepatic organoids were recognized as an effective tool for screening potential cures and modeling liver diseases effectively. The current review summarizes the importance of 3D hepatic organoid culture over other conventional 2D and 3D culture models and its applicability in Liver tissue engineering.
Keywords: 3D culture; hepatic function; hepatic organoids; hepatocytes; liver tissue engineering.
Conflict of interest statement
Authors declare no conflict of interest.
Figures
References
-
- Fernandez-Checa J.C., Bagnaninchi P., Ye H., Sancho-Bru P., Falcon-Perez J.M., Royo F., Garcia-Ruiz C., Konu O., Miranda J., Lunov O., et al. Advanced preclinical models for evaluation of drug-induced liver injury-consensus statement by the European Drug-Induced Liver Injury Network [PRO-EURO-DILI-NET] J. Hepatol. 2021;75:235–959. doi: 10.1016/j.jhep.2021.06.021. - DOI - PubMed
-
- Du Y., Li N., Long M. Chapter 6-Liver sinusoid on a chip. In: Doh J., Fletcher D., Piel M., editors. Methods in Cell Biology. Volume 146. Academic Press; Cambridge, MA, USA: 2018. pp. 105–134. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

