Nitrogenous Fertilizer Reduces Resistance but Enhances Tolerance to the Brown Planthopper in Fast-Growing, Moderately Resistant Rice
- PMID: 34821791
- PMCID: PMC8621593
- DOI: 10.3390/insects12110989
Nitrogenous Fertilizer Reduces Resistance but Enhances Tolerance to the Brown Planthopper in Fast-Growing, Moderately Resistant Rice
Abstract
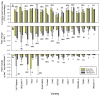
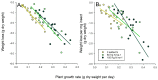
The brown planthopper, Nilaparvata lugens (Stål), is a key challenge to rice production in Asia. Outbreaks of planthoppers are associated with excessive fertilizer applications; consequently, we examined planthopper interactions with susceptible, tolerant and resistant varieties of rice under varying levels of soil nitrogen in a greenhouse experiment. We compared planthopper fitness (survival × reproduction) and plant tolerance (functional plant loss index) for 16 varieties at 0, 80 and 150 Kg added nitrogen ha-1. The planthoppers grew larger, developed more quickly and laid more eggs on susceptible varieties, compared with the resistant and tolerant varieties. Moreover, soil nitrogen generally increased planthopper fitness on resistant varieties, but relative resistance was maintained. Functional plant loss was highest among the susceptible varieties, but weight and growth rate reductions per mg of planthopper were often highest in the tolerant varieties. Tolerance was associated with large, fast-growing plants, with at least moderate resistance to the planthopper. Susceptibility was associated with a small size and/or an absence of resistance genes. Our results suggested that early-tillering rice plants can be both resistant and tolerant to the brown planthopper, but cannot be both susceptible and tolerant of planthoppers at high densities. This indicates that at least moderate resistance is required for tolerance against this herbivore. Furthermore, although dwarf varieties had a low tolerance of planthoppers, they could express resistance through functioning resistance genes.
Keywords: BPH3; BPH32; Nilaparvata lugens; Sogatella furcifera; compensation; phenotyping; phloem feeding; sustainable agriculture; xylem.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Smith C.M. Plant Resistance to Arthropods: Molecular and Conventional Approaches. Springer Science & Business Media; Berlin/Heidelberg, Germany: 2005.
-
- Horgan F.G. Diversity and defence: Plant-herbivore interactions at multiple scales and trophic levels. In: Gurr G.M., Wratten S.D., Snyder W.E., Read D.M.Y., editors. Biodiversity and Insect Pests: Key Issues for Sustainable Management. 1st ed. John Wiley & Sons; Oxford, UK: 2012. pp. 421–458.
-
- Bari A., Street K., Mackay M., Endresen D.T.F., De Pauw E., Ahmed A. Focused identification of germplasm strategy (FIGS) detects wheat stem rust resistance linked to environmental variables. Genet. Resour. Crop. Evol. 2012;59:1465–1481. doi: 10.1007/s10722-011-9775-5. - DOI
-
- Flanders K.L., Radcliffe E.B., Hawkes J.G. Geographic distribution of insect resistance in potatoes. Euphytica. 1997;93:201–221. doi: 10.1023/A:1002921315283. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

