Population Pharmacokinetics and Exposure-Response Analyses for Venetoclax in Combination with R-CHOP in Relapsed/Refractory and Previously Untreated Patients with Diffuse Large B Cell Lymphoma
- PMID: 34822104
- PMCID: PMC8799559
- DOI: 10.1007/s12325-021-01919-z
Population Pharmacokinetics and Exposure-Response Analyses for Venetoclax in Combination with R-CHOP in Relapsed/Refractory and Previously Untreated Patients with Diffuse Large B Cell Lymphoma
Abstract
Introduction: Outcomes remain poor in patients with diffuse large B cell lymphoma (DLBCL) who overexpress BCL-2 protein. We present population pharmacokinetics (PopPK) and exposure-response (ER) analyses for venetoclax (a selective BCL-2 inhibitor) administered with rituximab-cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in patients with relapsed/refractory (R/R) and previously untreated (1L) non-Hodgkin lymphoma (NHL) from the phase 1b/2 CAVALLI study, to confirm dose selection for future studies.
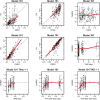
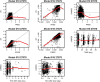
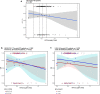
Methods: Analyses included 216 patients with R/R or 1L NHL treated for eight 21-day cycles with 400-800 mg venetoclax (cycle 1: days 4-10; cycles 2-8: days 1-10) in combination with R for eight cycles and CHOP for 6-8 cycles. A legacy PopPK model for venetoclax was used to describe the observed data and provide post hoc PK parameters. Venetoclax steady-state exposure (AUCss) was used to predict clinical efficacy, safety, or tolerability. To isolate the effect of venetoclax, ER analyses referenced data from the R-CHOP arm of a historical control study, GOYA, in 1L DLBCL.
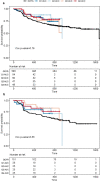
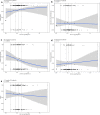
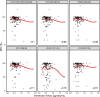
Results: There was no significant association between venetoclax AUCss and progression-free survival or complete response either for all-comers or the BCL-2-immunohistochemistry-positive subpopulation. No statistically significant trends were observed with venetoclax AUCss and the key grade ≥ 3 adverse events and serious adverse events. Similar dose intensities were observed for venetoclax and R-CHOP components across venetoclax exposures, suggesting venetoclax did not impact delivery of the R-CHOP backbone.
Conclusions: The PopPK and ER analyses, in addition to the positive benefit-risk observed in the clinical data, support the selection of 800 mg venetoclax given with R-CHOP for future studies in BCL-2-immunohistochemistry-positive patients with 1L DLBCL.
Trial registration: ClinicalTrials.gov Identifier NCT02055820.
Keywords: Diffuse Large B cell Lymphoma; Lymphoma; Pharmacokinetics; R-CHOP; Venetoclax.
© 2021. The Author(s).
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

