An update on the global use of risk assessment models and thromboprophylaxis in hospitalized patients with medical illnesses from the World Thrombosis Day steering committee: Systematic review and meta-analysis
- PMID: 34822215
- PMCID: PMC9299991
- DOI: 10.1111/jth.15607
An update on the global use of risk assessment models and thromboprophylaxis in hospitalized patients with medical illnesses from the World Thrombosis Day steering committee: Systematic review and meta-analysis
Abstract
Introduction: Venous thromboembolism (VTE) is a leading cause of cardiovascular morbidity and mortality. The majority of VTE events are hospital-associated. In 2008, the Epidemiologic International Day for the Evaluation of Patients at Risk for Venous Thromboembolism in the Acute Hospital Care Setting (ENDORSE) multinational cross-sectional study reported that only approximately 40% of medical patients at risk of VTE received adequate thromboprophylaxis.
Methods: In our systematic review and meta-analysis, we aimed at providing updated figures concerning the use of thromboprophylaxis globally. We focused on: (a) the frequency of patients with an indication to thromboprophylaxis according with individual models; (b) the use of adequate thromboprophylaxis; and (c) reported contraindications to thromboprophylaxis. Observational nonrandomized studies or surveys focusing on medically ill patients were considered eligible.
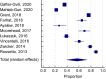
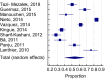
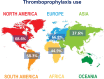
Results: After screening, we included 27 studies from 20 countries for a total of 137 288 patients. Overall, 50.5% (95% confidence interval [CI]: 41.9-59.1, I2 99%) of patients had an indication to thromboprophylaxis: of these, 54.5% (95% CI: 46.2-62.6, I2 99%) received adequate thromboprophylaxis. The use of adequate thromboprophylaxis was 66.8% in Europe (95% CI: 50.7-81.1, I2 98%), 44.9% in Africa (95% CI: 31.8-58.4, I2 96%), 37.6% in Asia (95% CI: 25.7-50.3, I2 97%), 58.3% in South America (95% CI: 31.1-83.1, I2 99%), and 68.6% in North America (95% CI: 64.9-72.6, I2 96%). No major differences in adequate thromboprophylaxis use were found across risk assessment models. Bleeding, thrombocytopenia, and renal/hepatic failure were the most frequently reported contraindications to thromboprophylaxis.
Conclusions: The use of anticoagulants for VTE prevention has been proven effective and safe, but thromboprophylaxis prescriptions are still unsatisfactory among hospitalized medically ill patients around the globe with marked geographical differences.
Keywords: World Thrombosis Day; epidemiology; thromboprophylaxis; thrombosis; venous thromboembolism.
© 2021 The Authors. Journal of Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis.
Conflict of interest statement
Dr. Barco has received honoraria from Boston Scientific, Concept Medical, INARI, Bayer, LeoPharma; institutional grants from Sanofi, Bard, Bentley, Concept Medical, Boston Scientific, Concept Medical; and support for congress participation and travel from Daiichi Sankyo and Bayer. Dr. Áinle: research grants (paid to university): Bayer, Daiichi‐Sankyo, Actelion, Leo Pharma. Dr. Ageno has received research support from Bayer and honoraria from Aspen, Bayer, BMS/Pfizer, Leo Pharma, Norgine, Sanofi, Daiichi Sankyo. Dr. Castellucci has received honoraria from Bayer, BMS‐Pfizer Alliance, The Academy, LEO Pharma, Sanofi, and Servier; she holds a Heart and Stroke Foundation of Canada National New Investigator Award, and a Tier 2 research Chair in Thrombosis and Anticoagulation Safety from the University of Ottawa. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res. 2016;118(9):1340‐1347. - PubMed
-
- Barco S, Woersching AL, Spyropoulos AC, Piovella F, Mahan CE. European Union‐28: an annualised cost‐of‐illness model for venous thromboembolism. Thromb Haemost. 2016;115(4):800‐808. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

