Short-course daily isoniazid and rifapentine for latent tuberculosis infection in people living with HIV who received coformulated bictegravir/emtricitabine/tenofovir alafenamide
- PMID: 34822220
- PMCID: PMC8614225
- DOI: 10.1002/jia2.25844
Short-course daily isoniazid and rifapentine for latent tuberculosis infection in people living with HIV who received coformulated bictegravir/emtricitabine/tenofovir alafenamide
Abstract
Introduction: Short-course preventive therapy with 1-month course of daily administration of isoniazid (300-mg) plus rifapentine (600-mg) (1HP) and 3-month course of weekly administration of isoniazid (900-mg) plus rifapentine (900-mg) (3HP) has higher completion rates than 9-month course of daily isoniazid (9H) for individuals with latent tuberculosis infection (LTBI). We aimed to evaluate the effect, safety and tolerability of 1HP in people living with HIV (PLWH) and LTBI who received coformulated bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF).
Methods: PLWH testing positive by interferon-gamma release assay and having received BIC/FTC/TAF for >2 weeks with plasma HIV RNA load (PVL) <200 copies/ml were enrolled. BIC trough plasma concentrations and cytokine profiles were determined before the first dose (day 1/baseline), 24 h after the 14th (day 15) and 28th (day 29) doses of 1HP. PVL were determined on days 15 and 29 of 1HP and every 3 months subsequently after discontinuation of 1HP.
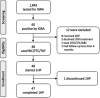
Results: From November 2019 to December 2020, 48 PLWH with LTBI were enrolled. One participant (2.1%) discontinued 1HP on day 15 due to fever and generalized rashes with PVL of 72 copies/ml, which was <50 copies/ml in three subsequent determinations while on BIC/FTC/TAF over the 12 months of follow-up. The percentages of BIC trough plasma concentrations above the protein-adjusted 95% effective concentration (paEC95 = 162 ng/ml) were 56.3% and 37.0% on days 15 and 29, respectively. The percentage of PVL <200 copies/ml was 91.7% on day 15, 97.8% on day 29 and 100% at both months 3 and 6. After a median observation of 52 weeks (interquartile range, 51-55), all participants continued BIC/FTC/TAF with a median PVL of 20 copies/ml (range 20-331). Except for the participant who discontinued 1HP because of allergic reactions, none of the participants had relevant symptoms or increases of the cytokine levels assessed between baseline and days 15 and 29 of 1HP.
Conclusions: BIC/FTC/TAF in combination with 1HP was well tolerated with a high completion rate. BIC trough plasma concentrations were significantly decreased with concurrent use of 1HP among PLWH with LTBI. While transient viral blips were observed during 1HP without causing subsequent treatment failure, such combination should be applied with caution.
Keywords: drug-drug interaction; integrase strand transfer inhibitor; protein-adjusted 95% effective concentration; rifamycin; trough concentration.
© 2021 The Authors. Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of the International AIDS Society.
Conflict of interest statement
C‐CH has received research support from Janssen, Merck, Gilead Sciences and ViiV and speaker honoraria from Abbvie, Bristol‐Myers Squibb, Gilead Sciences and ViiV, and served on advisory boards for Gilead Sciences, Janssen, ViiV and Abbvie. H‐YS has received research support from Gilead. Other authors have no competing interest to disclose.
Figures
References
-
- World Health Organization . Global Tuberculosis Report 2020. Available at: https://www.who.int/publications/i/item/9789240013131. Accessed 1 February 2021.
-
- World Health Organization . Latent TB infection: updated and consolidated guidelines for programmatic management. Available at: https://www.who.int/publications‐detail‐redirect/9789241550239. Accessed 1 February 2021. - PubMed
-
- Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, Ouassa T, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808–22. - PubMed
-
- Badje A, Moh R, Gabillard D, Guéhi C, Kabran M, Ntakpé JB, et al. Effect of isoniazid preventive therapy on risk of death in west African, HIV‐infected adults with high CD4 cell counts: long‐term follow‐up of the Temprano ANRS 12136 trial. Lancet Glob Health. 2017;5:e1080–e9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

