Heptapeptide Isolated from Isochrysis zhanjiangensis Exhibited Anti-Photoaging Potential via MAPK/AP-1/MMP Pathway and Anti-Apoptosis in UVB-Irradiated HaCaT Cells
- PMID: 34822497
- PMCID: PMC8625372
- DOI: 10.3390/md19110626
Heptapeptide Isolated from Isochrysis zhanjiangensis Exhibited Anti-Photoaging Potential via MAPK/AP-1/MMP Pathway and Anti-Apoptosis in UVB-Irradiated HaCaT Cells
Abstract
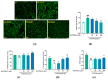
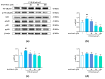
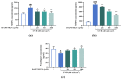
Marine microalgae can be used as sustainable protein sources in many fields with positive effects on human and animal health. DAPTMGY is a heptapeptide isolated from Isochrysis zhanjiangensis which is a microalga. In this study, we evaluated its anti-photoaging properties and mechanism of action in human immortalized keratinocytes cells (HaCaT). The results showed that DAPTMGY scavenged reactive oxygen species (ROS) and increase the level of endogenous antioxidants. In addition, through the exploration of its mechanism, it was determined that DAPIMGY exerted anti-photoaging effects. Specifically, the heptapeptide inhibits UVB-induced apoptosis through down-regulation of p53, caspase-8, caspase-3 and Bax and up-regulation of Bcl-2. Thus, DAPTMGY, isolated from I. zhanjiangensis, exhibits protective effects against UVB-induced damage.
Keywords: MMPs; UVB; apoptosis; heptapeptide; photoaging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Parrado C., Mascaraque M., Gilaberte Y., Juarranz A., Gonzalez S. Fernblock (Polypodium leucotomos Extract): Molecular Mechanisms and Pleiotropic Effects in Light-Related Skin Conditions, Photoaging and Skin Cancers, a Review. Int. J. Mol. Sci. 2016;17:1026. doi: 10.3390/ijms17071026. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

