The Two Sweet Sides of Janus Lectin Drive Crosslinking of Liposomes to Cancer Cells and Material Uptake
- PMID: 34822576
- PMCID: PMC8620536
- DOI: 10.3390/toxins13110792
The Two Sweet Sides of Janus Lectin Drive Crosslinking of Liposomes to Cancer Cells and Material Uptake
Abstract
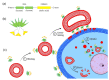
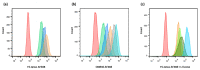
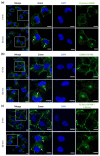
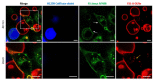
A chimeric, bispecific Janus lectin has recently been engineered with different, rationally oriented recognition sites. It can bind simultaneously to sialylated and fucosylated glycoconjugates. Because of its multivalent architecture, this lectin reaches nanomolar avidities for sialic acid and fucose. The lectin was designed to detect hypersialylation-a dysregulation in physiological glycosylation patterns, which promotes the tumor growth and progression of several cancer types. In this study, the characteristic properties of this bispecific Janus lectin were investigated on human cells by flow cytometry and confocal microscopy in order to understand the fundamentals of its interactions. We evaluated its potential in targeted drug delivery, precisely leading to the cellular uptake of liposomal content in human epithelial cancer cells. We successfully demonstrated that Janus lectin mediates crosslinking of glyco-decorated giant unilamellar vesicles (GUVs) and H1299 lung epithelial cells. Strikingly, the Janus lectin induced the internalization of liposomal lipids and also of complete GUVs. Our findings serve as a solid proof of concept for lectin-mediated targeted drug delivery using glyco-decorated liposomes as possible drug carriers to cells of interest. The use of Janus lectin for tumor recognition certainly broadens the possibilities for engineering diverse tailor-made lectin constructs, specifically targeting extracellular structures of high significance in pathological conditions.
Keywords: cancer cell targeting; chimeric carbohydrate; drug delivery; giant unilamellar vesicles; hypersialylation; live-cell imaging; protein engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

