Novel drug developmental strategies for treatment-resistant depression
- PMID: 34822719
- PMCID: PMC9303797
- DOI: 10.1111/bph.15753
Novel drug developmental strategies for treatment-resistant depression
Abstract
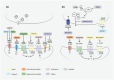
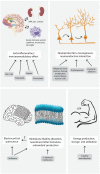
Major depressive disorder is a leading cause of disability worldwide. Because conventional therapies are ineffective in many patients, novel strategies are needed to overcome treatment-resistant depression (TRD). Limiting factors of successful drug development in the last decades were the lack of (1) knowledge of pathophysiology, (2) translational animal models and (3) objective diagnostic biomarkers. Here, we review novel drug targets and drug candidates currently investigated in Phase I-III clinical trials. The most promising approaches are inhibition of glutamatergic neurotransmission by NMDA and mGlu5 receptor antagonists, modulation of the opioidergic system by κ receptor antagonists, and hallucinogenic tryptamine derivates. The only registered drug for TRD is the NMDA receptor antagonist, S-ketamine, but add-on therapies with second-generation antipsychotics, certain nutritive, anti-inflammatory and neuroprotective agents seem to be effective. Currently, there is an intense research focus on large-scale, high-throughput omics and neuroimaging studies. These results might provide new insights into molecular mechanisms and potential novel therapeutic strategies.
Keywords: antidepressant; glutamate; monoamine; neuroimaging; neuroinflammation; neuroplasticity; opioid.
© 2021 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Alexander, S. P. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Abbracchio, M. P. , Alexander, W. , Al‐Hosaini, K. , Bäck, M. , Barnes, N. M. , Bathgate, R. , … Ye, R. D. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: G protein‐coupled receptors. British Journal of Pharmacology, 178(Suppl 1), S27–S156. 10.1111/bph.15538 - DOI - PubMed
-
- Alexander, S. P. , Cidlowski, J. A. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Coons, L. , Fuller, P. J. , Korach, K. S. , & Young, M. J. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Nuclear hormone receptors. British Journal of Pharmacology, 178(S1), S246–S263. 10.1111/bph.15540 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

