Chronic wasting disease in Europe: new strains on the horizon
- PMID: 34823556
- PMCID: PMC8613970
- DOI: 10.1186/s13028-021-00606-x
Chronic wasting disease in Europe: new strains on the horizon
Abstract
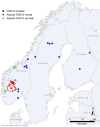
Prion diseases are fatal neurodegenerative disorders with known natural occurrence in humans and a few other mammalian species. The diseases are experimentally transmissible, and the agent is derived from the host-encoded cellular prion protein (PrPC), which is misfolded into a pathogenic conformer, designated PrPSc (scrapie). Aggregates of PrPSc molecules, constitute proteinaceous infectious particles, known as prions. Classical scrapie in sheep and goats and chronic wasting disease (CWD) in cervids are known to be infectious under natural conditions. In CWD, infected animals can shed prions via bodily excretions, allowing direct host-to-host transmission or indirectly via prion-contaminated environments. The robustness of prions means that transmission via the latter route can be highly successful and has meant that limiting the spread of CWD has proven difficult. In 2016, CWD was diagnosed for the first time in Europe, in reindeer (Rangifer tarandus) and European moose (Alces alces). Both were diagnosed in Norway, and, subsequently, more cases were detected in a semi-isolated wild reindeer population in the Nordfjella area, in which the first case was identified. This population was culled, and all reindeer (approximately 2400) were tested for CWD; 18 positive animals, in addition to the first diagnosed case, were found. After two years and around 25,900 negative tests from reindeer (about 6500 from wild and 19,400 from semi-domesticated) in Norway, a new case was diagnosed in a wild reindeer buck on Hardangervidda, south of the Nordfjella area, in 2020. Further cases of CWD were also identified in moose, with a total of eight in Norway, four in Sweden, and two cases in Finland. The mean age of these cases is 14.7 years, and the pathological features are different from North American CWD and from the Norwegian reindeer cases, resembling atypical prion diseases such as Nor98/atypical scrapie and H- and L-forms of BSE. In this review, these moose cases are referred to as atypical CWD. In addition, two cases were diagnosed in red deer (Cervus elaphus) in Norway. The emergence of CWD in Europe is a threat to European cervid populations, and, potentially, a food-safety challenge, calling for a swift, evidence-based response. Here, we review data on surveillance, epidemiology, and disease characteristics, including prion strain features of the newly identified European CWD agents.
Keywords: CWD; Deer; Fennoscandia; Moose; Nordic countries; Prion disease; Red deer; Reindeer.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

