PINK1 deficiency impairs osteoblast differentiation through aberrant mitochondrial homeostasis
- PMID: 34823575
- PMCID: PMC8614054
- DOI: 10.1186/s13287-021-02656-4
PINK1 deficiency impairs osteoblast differentiation through aberrant mitochondrial homeostasis
Abstract
Background: PTEN-induced kinase 1 (PINK1) is a serine/threonine-protein kinase in mitochondria that is critical for mitochondrial quality control. PINK1 triggers mitophagy, a selective autophagy of mitochondria, and is involved in mitochondrial regeneration. Although increments of mitochondrial biogenesis and activity are known to be crucial during differentiation, data regarding the specific role of PINK1 in osteogenic maturation and bone remodeling are limited.
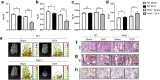
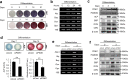
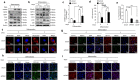
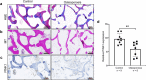
Methods: We adopted an ovariectomy model in female wildtype and Pink1-/- mice. Ovariectomized mice were analyzed using micro-CT, H&E staining, Masson's trichrome staining. RT-PCR, western blot, immunofluorescence, alkaline phosphatase, and alizarin red staining were performed to assess the expression of PINK1 and osteogenic markers in silencing of PINK1 MC3T3-E1 cells. Clinical relevance of PINK1 expression levels was determined via qRT-PCR analysis in normal and osteoporosis patients.
Results: A significant decrease in bone mass and collagen deposition was observed in the femurs of Pink1-/- mice after ovariectomy. Ex vivo, differentiation of osteoblasts was inhibited upon Pink1 downregulation, accompanied by impaired mitochondrial homeostasis, increased mitochondrial reactive oxygen species production, and defects in mitochondrial calcium handling. Furthermore, PINK1 expression was reduced in bones from patients with osteoporosis, which supports the practical role of PINK1 in human bone disease.
Conclusions: In this study, we demonstrated that activation of PINK1 is a requisite in osteoblasts during differentiation, which is related to mitochondrial quality control and low reactive oxygen species production. Enhancing PINK1 activity might be a possible treatment target in bone diseases as it can promote a healthy pool of functional mitochondria in osteoblasts.
Keywords: Mitochondria; Osteogenesis; Osteoporosis; PINK1.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

