Brain-derived estrogen and neural function
- PMID: 34823913
- PMCID: PMC8816863
- DOI: 10.1016/j.neubiorev.2021.11.014
Brain-derived estrogen and neural function
Abstract
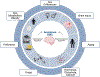
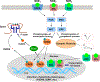
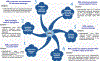
Although classically known as an endocrine signal produced by the ovary, 17β-estradiol (E2) is also a neurosteroid produced in neurons and astrocytes in the brain of many different species. In this review, we provide a comprehensive overview of the localization, regulation, sex differences, and physiological/pathological roles of brain-derived E2 (BDE2). Much of what we know regarding the functional roles of BDE2 has come from studies using specific inhibitors of the E2 synthesis enzyme, aromatase, as well as the recent development of conditional forebrain neuron-specific and astrocyte-specific aromatase knockout mouse models. The evidence from these studies support a critical role for neuron-derived E2 (NDE2) in the regulation of synaptic plasticity, memory, socio-sexual behavior, sexual differentiation, reproduction, injury-induced reactive gliosis, and neuroprotection. Furthermore, we review evidence that astrocyte-derived E2 (ADE2) is induced following brain injury/ischemia, and plays a key role in reactive gliosis, neuroprotection, and cognitive preservation. Finally, we conclude by discussing the key controversies and challenges in this area, as well as potential future directions for the field.
Keywords: 17β-Estradiol; Aromatase; Gliosis; Memory; Neuroprotection; Neurosteroid; Sexual behavior; Synaptic plasticity.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors report no declarations of interest.
Figures

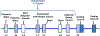



References
-
- Abdelgadir SE, Resko JA, Ojeda SR, Lephart ED, McPhaul MJ, Roselli CE, 1994. Androgens regulate aromatase cytochrome P450 messenger ribonucleic acid in rat brain. Endocrinology 135, 395–401. - PubMed
-
- Amodio R, Esposito E, De Ruvo C, Bellavia V, Amodio E, Carruba G, 2006. Red wine extract prevents neuronal apoptosis in vitro and reduces mortality of transgenic mice. Ann. N. Y. Acad. Sci 1089, 88–97. - PubMed
-
- Arevalo MA, Azcoitia I, Garcia-Segura LM, 2015. The neuroprotective actions of oestradiol and oestrogen receptors. Nat. Rev. Neurosci 16, 17–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

