Broadening symptom criteria improves early case identification in SARS-CoV-2 contacts
- PMID: 34824057
- PMCID: PMC8620106
- DOI: 10.1183/13993003.02308-2021
Broadening symptom criteria improves early case identification in SARS-CoV-2 contacts
Abstract
Background: The success of case isolation and contact tracing for the control of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission depends on the accuracy and speed of case identification. We assessed whether inclusion of additional symptoms alongside three canonical symptoms (CS), i.e. fever, cough and loss or change in smell or taste, could improve case definitions and accelerate case identification in SARS-CoV-2 contacts.
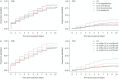
Methods: Two prospective longitudinal London (UK)-based cohorts of community SARS-CoV-2 contacts, recruited within 5 days of exposure, provided independent training and test datasets. Infected and uninfected contacts completed daily symptom diaries from the earliest possible time-points. Diagnostic information gained by adding symptoms to the CS was quantified using likelihood ratios and area under the receiver operating characteristic curve. Improvements in sensitivity and time to detection were compared with penalties in terms of specificity and number needed to test.
Results: Of 529 contacts within two cohorts, 164 (31%) developed PCR-confirmed infection and 365 (69%) remained uninfected. In the training dataset (n=168), 29% of infected contacts did not report the CS. Four symptoms (sore throat, muscle aches, headache and appetite loss) were identified as early-predictors (EP) which added diagnostic value to the CS. The broadened symptom criterion "≥1 of the CS, or ≥2 of the EP" identified PCR-positive contacts in the test dataset on average 2 days earlier after exposure (p=0.07) than "≥1 of the CS", with only modest reduction in specificity (5.7%).
Conclusions: Broadening symptom criteria to include individuals with at least two of muscle aches, headache, appetite loss and sore throat identifies more infections and reduces time to detection, providing greater opportunities to prevent SARS-CoV-2 transmission.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: No competing interests were declared by any of the study authors.
Figures




References
-
- Singanayagam A, Hakki S, Dunning J, et al. . Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis 2022; 22: 183–195. doi:10.1016/S1473-3099(21)00648-4 - DOI - PMC - PubMed
-
- World Health Organization . Contact tracing in the context of COVID-19. 2021. www.who.int/publications/i/item/contact-tracing-in-the-context-of-covid-19 Date last accessed: 26 November 2021.
-
- Kucharski AJ, Klepac P, Conlan AJK, et al. . Effectiveness of isolation, testing, contact tracing, and physical distancing on reducing transmission of SARS-CoV-2 in different settings: a mathematical modelling study. Lancet Infect Dis 2020; 20: 1151–1160. doi:10.1016/S1473-3099(20)30457-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
