Implementation of an early rule-out pathway for myocardial infarction using a high-sensitivity cardiac troponin T assay
- PMID: 34824100
- PMCID: PMC8627412
- DOI: 10.1136/openhrt-2021-001769
Implementation of an early rule-out pathway for myocardial infarction using a high-sensitivity cardiac troponin T assay
Abstract
Objectives: Patients with suspected acute coronary syndrome and high-sensitivity cardiac troponin (hs-cTn) concentrations below the limit of detection at presentation are low risk. We aim to determine whether implementing this approach facilitates the safe early discharge of patients.
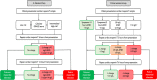
Methods: In a prospective single-centre cohort study, consecutive patients with suspected acute coronary syndrome were included before (standard care) and after (intervention) implementation of an early rule-out pathway. During standard care, myocardial infarction was ruled out if hs-cTnT concentrations were <99th centile (14 ng/L) at presentation and at 6-12 hours after symptom onset. In the intervention, patients were ruled out if hs-cTnT concentrations were <5 ng/L at presentation and symptoms present for ≥3 hours or were ≥5 ng/L and unchanged within the reference range at 3 hours. We compared duration of stay (efficacy) and all-cause death at 1 year (safety) before and after implementation.
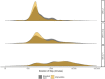
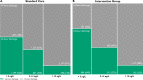
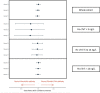
Results: We included 10 315 consecutive patients (64±16 years, 46% women) with 6642 (64%) and 3673 (36%) in the standard care and intervention groups, respectively. Duration of stay was reduced from 534 (IQR, 220-2279) to 390 (IQR, 218-1910) min (p<0.001) after implementation. At 1 year, all-cause death occurred in 10.9% (721 of 6642) and 10.4% (381 of 3673) of patients in the standard care group (referent) and intervention group, respectively (adjusted OR 1.02, 95% CI 0.88 to 1.18).
Conclusion: In patients with suspected acute coronary syndrome, implementing an early rule-out pathway using hs-cTnT concentrations <5 ng/L at presentation reduced the duration of stay in hospital without compromising safety.
Keywords: acute coronary syndrome; biomarkers; chest pain.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: DS reports that NHS Fife received a small research grant from Roche Diagnostics to support data linkage. NLM reports research grants awarded to the University of Edinburgh from Abbott Diagnostics and Siemens Healthineers outside the submitted work, and honoraria from Abbott Diagnostics, Siemens Healthineers, Roche Diagnostics and LumiraDx. The other authors declared no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
