The JAK/STAT signaling pathway: from bench to clinic
- PMID: 34824210
- PMCID: PMC8617206
- DOI: 10.1038/s41392-021-00791-1
The JAK/STAT signaling pathway: from bench to clinic
Abstract
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway was discovered more than a quarter-century ago. As a fulcrum of many vital cellular processes, the JAK/STAT pathway constitutes a rapid membrane-to-nucleus signaling module and induces the expression of various critical mediators of cancer and inflammation. Growing evidence suggests that dysregulation of the JAK/STAT pathway is associated with various cancers and autoimmune diseases. In this review, we discuss the current knowledge about the composition, activation, and regulation of the JAK/STAT pathway. Moreover, we highlight the role of the JAK/STAT pathway and its inhibitors in various diseases.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
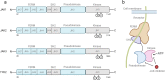



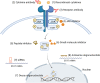
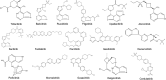
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

