Structures and functions of coronavirus replication-transcription complexes and their relevance for SARS-CoV-2 drug design
- PMID: 34824452
- PMCID: PMC8613731
- DOI: 10.1038/s41580-021-00432-z
Structures and functions of coronavirus replication-transcription complexes and their relevance for SARS-CoV-2 drug design
Abstract
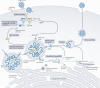
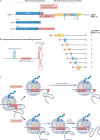
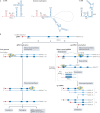
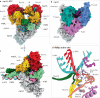
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has killed millions of people and continues to cause massive global upheaval. Coronaviruses are positive-strand RNA viruses with an unusually large genome of ~30 kb. They express an RNA-dependent RNA polymerase and a cohort of other replication enzymes and supporting factors to transcribe and replicate their genomes. The proteins performing these essential processes are prime antiviral drug targets, but drug discovery is hindered by our incomplete understanding of coronavirus RNA synthesis and processing. In infected cells, the RNA-dependent RNA polymerase must coordinate with other viral and host factors to produce both viral mRNAs and new genomes. Recent research aiming to decipher and contextualize the structures, functions and interplay of the subunits of the SARS-CoV-2 replication and transcription complex proteins has burgeoned. In this Review, we discuss recent advancements in our understanding of the molecular basis and complexity of the coronavirus RNA-synthesizing machinery. Specifically, we outline the mechanisms and regulation of RNA translation, replication and transcription. We also discuss the composition of the replication and transcription complexes and their suitability as targets for antiviral therapy.
© 2021. Springer Nature Limited.
Conflict of interest statement
E.A.C has received funding from Gilead Sciences to fund research on remdesivir’s incorporation into the RNA-dependent RNA polymerase. The other authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

