γ-Glutamylcysteine Alleviates Ischemic Stroke-Induced Neuronal Apoptosis by Inhibiting ROS-Mediated Endoplasmic Reticulum Stress
- PMID: 34824669
- PMCID: PMC8610689
- DOI: 10.1155/2021/2961079
γ-Glutamylcysteine Alleviates Ischemic Stroke-Induced Neuronal Apoptosis by Inhibiting ROS-Mediated Endoplasmic Reticulum Stress
Abstract
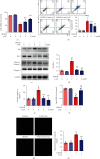
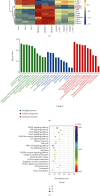
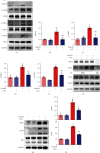
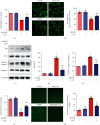
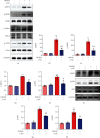
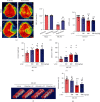
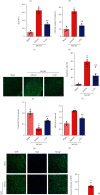
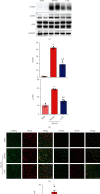
Ischemic stroke is a severe and acute neurological disorder with limited therapeutic strategies currently available. Oxidative stress is one of the critical pathological factors in ischemia/reperfusion injury, and high levels of reactive oxygen species (ROS) may drive neuronal apoptosis. Rescuing neurons in the penumbra is a potential way to recover from ischemic stroke. Endogenous levels of the potent ROS quencher glutathione (GSH) decrease significantly after cerebral ischemia. Here, we aimed to investigate the neuroprotective effects of γ-glutamylcysteine (γ-GC), an immediate precursor of GSH, on neuronal apoptosis and brain injury during ischemic stroke. Middle cerebral artery occlusion (MCAO) and oxygen-glucose deprivation/reoxygenation (OGD/R) were used to mimic cerebral ischemia in mice, neuronal cell lines, and primary neurons. Our data indicated that exogenous γ-GC treatment mitigated oxidative stress, as indicated by upregulated GSH and decreased ROS levels. In addition, γ-GC attenuated ischemia/reperfusion-induced neuronal apoptosis and brain injury in vivo and in vitro. Furthermore, transcriptomics approaches and subsequent validation studies revealed that γ-GC attenuated penumbra neuronal apoptosis by inhibiting the activation of protein kinase R-like endoplasmic reticulum kinase (PERK) and inositol-requiring enzyme 1α (IRE1α) in the endoplasmic reticulum (ER) stress signaling pathway in OGD/R-treated cells and ischemic brain tissues. To the best of our knowledge, this study is the first to report that γ-GC attenuates ischemia-induced neuronal apoptosis by suppressing ROS-mediated ER stress. γ-GC may be a promising therapeutic agent for ischemic stroke.
Copyright © 2021 Hui-qin Li et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Sappanone A Protects Against Inflammation, Oxidative Stress and Apoptosis in Cerebral Ischemia-Reperfusion Injury by Alleviating Endoplasmic Reticulum Stress.Inflammation. 2021 Jun;44(3):934-945. doi: 10.1007/s10753-020-01388-6. Epub 2021 Jan 7. Inflammation. 2021. PMID: 33411101
-
Phelligridimer A enhances the expression of mitofusin 2 and protects against cerebral ischemia/reperfusion injury.Chem Biol Interact. 2024 Aug 1;398:111090. doi: 10.1016/j.cbi.2024.111090. Epub 2024 May 31. Chem Biol Interact. 2024. PMID: 38825057
-
Ginsenoside Rb1 reduced ischemic stroke-induced apoptosis through endoplasmic reticulum stress-associated IRE1/TRAF2/JNK pathway.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jan;398(1):747-764. doi: 10.1007/s00210-024-03292-4. Epub 2024 Jul 25. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39052059
-
Developing targeted antioxidant nanomedicines for ischemic penumbra: Novel strategies in treating brain ischemia-reperfusion injury.Redox Biol. 2024 Jul;73:103185. doi: 10.1016/j.redox.2024.103185. Epub 2024 May 7. Redox Biol. 2024. PMID: 38759419 Free PMC article. Review.
-
The role of oxidative stress in blood-brain barrier disruption during ischemic stroke: Antioxidants in clinical trials.Biochem Pharmacol. 2024 Oct;228:116186. doi: 10.1016/j.bcp.2024.116186. Epub 2024 Mar 30. Biochem Pharmacol. 2024. PMID: 38561092 Review.
Cited by
-
Sodium formononetin-3'-sulphonate alleviates cerebral ischemia-reperfusion injury in rats via suppressing endoplasmic reticulum stress-mediated apoptosis.BMC Neurosci. 2022 Dec 9;23(1):74. doi: 10.1186/s12868-022-00762-4. BMC Neurosci. 2022. PMID: 36482320 Free PMC article.
-
Inhibition of the Activating Transcription Factor 6 Branch of Endoplasmic Reticulum Stress Ameliorates Brain Injury after Deep Hypothermic Circulatory Arrest.J Clin Med. 2023 Jan 19;12(3):814. doi: 10.3390/jcm12030814. J Clin Med. 2023. PMID: 36769462 Free PMC article.
-
Cerebral Glucose Metabolism and Potential Effects on Endoplasmic Reticulum Stress in Stroke.Aging Dis. 2023 Apr 1;14(2):450-467. doi: 10.14336/AD.2022.0905. eCollection 2023 Apr 1. Aging Dis. 2023. PMID: 37008060 Free PMC article. Review.
-
Therapeutic Potential of Targeting the PERK Signaling Pathway in Ischemic Stroke.Pharmaceuticals (Basel). 2024 Mar 8;17(3):353. doi: 10.3390/ph17030353. Pharmaceuticals (Basel). 2024. PMID: 38543139 Free PMC article. Review.
-
MYPT1SMKO Mice Function as a Novel Spontaneous Age- and Hypertension-Dependent Animal Model of CSVD.Transl Stroke Res. 2024 Jun;15(3):606-619. doi: 10.1007/s12975-023-01142-8. Epub 2023 Feb 27. Transl Stroke Res. 2024. PMID: 36843141
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

