Tools and Methods for Investigating Synthetic Metal-Catalyzed Reactions in Living Cells
- PMID: 34824879
- PMCID: PMC8612649
- DOI: 10.1021/acscatal.1c00438
Tools and Methods for Investigating Synthetic Metal-Catalyzed Reactions in Living Cells
Abstract
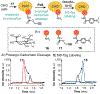
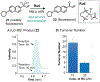
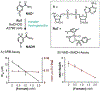
Although abiotic catalysts are capable of promoting numerous new-to-nature reactions, only a small subset has so far been successfully integrated into living systems. Research in intracellular catalysis requires an interdisciplinary approach that takes advantage of both chemical and biological tools as well as state-of-the-art instrumentations. In this perspective, we will focus on the techniques that have made studying metal-catalyzed reactions in cells possible using representative examples from the literature. Although the lack of quantitative data in vitro and in vivo has somewhat limited progress in the catalyst development process, recent advances in characterization methods should help overcome some of these deficiencies. Given its tremendous potential, we believe that intracellular catalysis will play a more prominent role in the development of future biotechnologies and therapeutics.
Keywords: bioassays; biocompatible; bioorthogonal; flow cytometry; intracellular; mass spectrometry; microscopy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

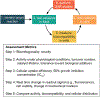





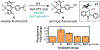

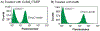
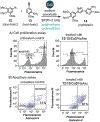







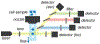


References
-
- Oparin AI The Origin of Life and the Origin of Enzymes. Adv. Enzymol. Relat. Areas Mol. Biol 1965, 347–380. - PubMed
-
- Schopf JW; Kudryavtsev AB; Czaja AD; Tripathi AB Evidence of Archean Life: Stromatolites and Microfossils. Precambrian Res. 2007, 158, 141–155.
-
- Robertson AJB The Early History of Catalysis. Platin. Met. Rev 1975, 19, 64–69.
-
- Hartwig JF Organotransition Metal Chemistry; University Science Books: Mill Valley, California, 2010.
Grants and funding
LinkOut - more resources
Full Text Sources
