C-H Bond Functionalization of Amines: A Graphical Overview of Diverse Methods
- PMID: 34825124
- PMCID: PMC8612105
- DOI: 10.1055/s-0040-1706051
C-H Bond Functionalization of Amines: A Graphical Overview of Diverse Methods
Abstract
This Graphical Review provides a concise overview of the manifold and mechanistically diverse methods that enable the functionalization of sp3 C-H bonds in amines and their derivatives.
Keywords: C–H bond functionalization; amines; catalysis; heterocycles; synthesis.
Conflict of interest statement
Conflict of Interest The authors declare no conflict of interest.
Figures



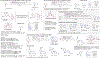




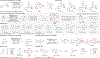















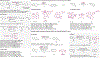















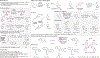
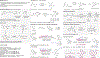





References
-
- Peterson DJ; Hays HR J. Org. Chem 1965, 30, 1939.
- Lepley AR; Giumanini AG J. Org. Chem 1966, 31, 2055.
- Ahlbrecht H; Dollinger H Tetrahedron Lett. 1984, 25, 1353.
- Gessner VH; Strohmann C J. Am. Chem. Soc 2008, 130, 14412. - PubMed
- Kessar SV; Singh P; Vohra R; Kaur NP; Singh KN J. Chem. Soc., Chem. Commun 1991, 568.
- Kessar SV; Vohra R; Kaur NP Tetrahedron Lett. 1991, 32, 3221.
- De Ceglie MC; Musio B; Affortunato F; Moliterni A; Altomare A; Florio S; Luisi R Chem. Eur. J 2011, 17, 286. - PubMed
- Singh KN; Singh P; Singh P; Deol YS Org. Lett 2012, 14, 2202. - PubMed
- Lepley AR; Khan WA J. Org. Chem 1966, 31, 2061.
- Lepley AR; Khan WA Chem. Commun 1967, 1198.
- Kessar SV; Singh P Chem. Rev 1997, 97, 721. - PubMed
- Katritzky AR; Qi M Tetrahedron 1998, 54, 2647.
- Ferey V; Toupet L; Le Gall T; Mioskowski C Angew. Chem., Int. Ed. Engl 1996, 35, 430.
- Vedejs E; Kendall JT J. Am. Chem. Soc 1997, 119, 6941.
- Ebden MR; Simpkins NS; Fox DNA Tetrahedron 1998, 54, 12923.
- Kessar SV; Singh P; Singh KN; Venugopalan P; Kaur A; Bharatam PV; Sharma AK J. Am. Chem. Soc 2007, 129, 4506. - PubMed
- Harmata M; Carter KW; Jones DE; Kahraman M Tetrahedron Lett. 1996, 37, 6267.
- Kovács E; Huszka B; Gáti T; Nyerges M; Faigl F; Mucsi Z J. Org. Chem 2019, 84, 7100. - PubMed
- Kovács E; Faigl F; Mucsi Z J. Org. Chem 2020, 85, 11226. - PMC - PubMed
-
- Keefer LK; Fodor CH J. Am. Chem. Soc 1970, 92, 5747.
- Seebach D; Enders D Angew. Chem., Int. Ed. Engl 1972, 11, 301.
- Seebach D; Enders D Angew. Chem., Int. Ed. Engl 1972, 11, 1101.
- Seebach D; Wykypiel W Synthesis 1979, 423.
- Seebach D; Enders D J. Med. Chem 1974, 17, 1225. - PubMed
- Fraser RR; Passannanti S Synthesis 1976, 540.
- Wykypiel W; Seebach D Tetrahedron Lett. 1980, 21, 1927.
- Savignac P; Dreux M; Leroux Y Tetrahedron Lett. 1974, 15, 2651.
- Savignac P; Leroux Y J. Organomet. Chem 1973, 57, C47.
- Magnus P; Roy G Synthesis 1980, 575.
- Seebach D; Yoshifuji M Helv. Chim. Acta 1981, 64, 643.
- Beak P; Zajdel WJ J. Am. Chem. Soc 1984, 106, 1010.
- Meyers AI; Edwards PD; Rieker WF; Bailey TR J. Am. Chem. Soc 1984, 106, 3270.
- Meyers AL; Dickman DA; Boes M Tetrahedron 1987, 43, 5095.
- Meyers AI Tetrahedron 1992, 48, 2589.
- Seebach D; Enders D Angew. Chem., Int. Ed. Engl 1975, 14, 15.
- Beak P; Reitz DB Chem. Rev 1978, 78, 275.
- Beak P; Zajdel WJ; Reitz DB Chem. Rev 1984, 84, 471.
- Clayden J Organolithiums: Selectivity for Synthesis, In Tetrahedron Organic Chemistry Series, Vol. 23; Clayden J, Ed.; Pergamon: Amsterdam, 2002, 9.
- Fraser RR; Boussard G; Postescu ID; Whiting JJ; Wigfield YY Can. J. Chem 1973, 51, 1109.
- Lyle RE; Saavedra JE; Lyle GG; Fribush HM; Marshall JL; Lijinsky W; Singer GM Tetrahedron Lett. 1976, 17, 4431.
- Seebach D; Lubosch W Angew. Chem., Int. Ed. Engl 1976, 15, 313.
- Seebach D; Hassel T Angew. Chem., Int. Ed. Engl 1978, 17, 274.
- Meyers AI; Ten Hoeve W J. Am. Chem. Soc 1980, 102, 7125.
- Seebach D; Lohmann J-J; Syfrig MA; Yoshifuji M Tetrahedron 1983, 39, 1963.
- Gawley RE; Hart G; Goicoechea-Pappas M; Smith AL J. Org. Chem 1986, 51, 3076.
- Gawley RE; Rein K; Chemburkar S J. Org. Chem 1989, 54, 3002.
- Meyers AI; Milot G J. Org. Chem 1993, 58, 6538.
- Nain Singh K; Singh P; Kaur A Synth. Commun 2006, 36, 3339.
-
- Beak P; Lee W-K Tetrahedron Lett. 1989, 30, 1197.
- Beak P; Lee WK J. Org. Chem 1990, 55, 2578.
- Beak P; Lee WK J. Org. Chem 1993, 58, 1109.
- Xiao D; Lavey BJ; Palani A; Wang C; Aslanian RG; Kozlowski JA; Shih N-Y; McPhail AT; Randolph GP; Lachowicz JE; Duffy RA Tetrahedron Lett. 2005, 46, 7653.
- Aeyad T; Williams JD; Meijer AJHM; Coldham I Synlett 2017, 28, 2765.
- Beak P; Wu S; Yum EK; Jun YM J. Org. Chem 1994, 59, 276.
- Dieter RK; Li S Tetrahedron Lett. 1995, 36, 3613.
- Dieter RK; Li S J. Org. Chem 1997, 62, 7726.
- Dieter RK; Dieter JW; Alexander CW; Bhinderwala NS J. Org. Chem 1996, 61, 2930. - PubMed
- Dieter RK; Velu SE J. Org. Chem 1997, 62, 3798. - PubMed
- Dieter RK; Lu K; Velu SE J. Org. Chem 2000, 65, 8715. - PubMed
- Barker G; O’Brien P; Campos KR Org. Lett 2010, 12, 4176. - PubMed
- Kwong A; Firth JD; Farmer TJ; O’Brien P Tetrahedron 2021, 81, 131899.
- Stead D; O’Brien P; Sanderson AJ Org. Lett 2005, 7, 4459. - PubMed
- Berkheij M; van der Sluis L; Sewing C; den Boer DJ; Terpstra JW; Hiemstra H; Iwema Bakker WI; van den Hoogenband A; van Maarseveen JH Tetrahedron Lett. 2005, 46, 2369.
- Hodgson DM; Humphreys PG; Xu Z; Ward JG Angew. Chem. Int. Ed 2007, 46, 2245. - PubMed
- Li X; Leonori D; Sheikh NS; Coldham I Chem. Eur. J 2013, 19, 7724. - PubMed
- Pizzuti MG; Minnaard AJ; Feringa BL Org. Biomol. Chem 2008, 6, 3464. - PubMed
- Krishnan S; Bagdanoff JT; Ebner DC; Ramtohul YK; Tambar UK; Stoltz BM J. Am. Chem. Soc 2008, 130, 13745. - PMC - PubMed
- Dieter RK; Sharma RR; Ryan W Tetrahedron Lett. 1997, 38, 783.
- Dieter RK; Lu K J. Org. Chem 2002, 67, 847. - PubMed
- Coldham I; Leonori D Org. Lett 2008, 10, 3923. - PubMed
-
- Kerrick ST; Beak P J. Am. Chem. Soc 1991, 113, 9708.
- Beak P; Kerrick ST; Wu S; Chu J J. Am. Chem. Soc 1994, 116, 3231.
- Dearden MJ; Firkin CR; Hermet J-PR; O’Brien P J. Am. Chem. Soc 2002, 124, 11870. - PubMed
- Campos KR; Klapars A; Waldman JH; Dormer PG; Chen C-Y J. Am. Chem. Soc 2006, 128, 3538. - PubMed
- Seel S; Thaler T; Takatsu K; Zhang C; Zipse H; Straub BF; Mayer P; Knochel P J. Am. Chem. Soc 2011, 133, 4774. - PubMed
- Kasten K; Seling N; O’Brien P Org. React 2019, 100, 255.
- Wong JYF; Barker G Tetrahedron 2020, 76, 131704.
- Gallagher DJ; Beak P J. Org. Chem 1995, 60, 7092.
- Wilkinson TJ; Stehle NW; Beak P Org. Lett 2000, 2, 155. - PubMed
- Phuan P-W; Ianni JC; Kozlowski MC J. Am. Chem. Soc 2004, 126, 15473. - PubMed
- Coldham I; Leonori D J. Org. Chem 2010, 75, 4069. - PubMed
- Sheikh NS; Leonori D; Barker G; Firth JD; Campos KR; Meijer AJHM; O’Brien P; Coldham I J. Am. Chem. Soc 2012, 134, 5300. - PubMed
- Kizirian J-C; Caille J-C; Alexakis A Tetrahedron Lett. 2003, 44, 8893.
- Hermet J-PR; Porter DW; Dearden MJ; Harrison JR; Koplin T; O’Brien P; Parmene J; Tyurin V; Whitwood AC; Gilday J; Smith NM Org. Biomol. Chem 2003, 1, 3977. - PubMed
- McGrath MJ; Bilke JL; O’Brien P Chem. Commun 2006, 2607. - PubMed
- McGrath MJ; O’Brien P J. Am. Chem. Soc 2005, 127, 16378. - PubMed
-
- Coldham I; Raimbault S; Whittaker DTE; Chovatia PT; Leonori D; Patel JJ; Sheikh NS Chem. Eur. J 2010, 16, 4082. - PubMed
- Millet A; Larini P; Clot E; Baudoin O Chem. Sci 2013, 4, 2241.
- Cordier CJ; Lundgren RJ; Fu GC J. Am. Chem. Soc 2013, 135, 10946. - PMC - PubMed
- Mu X; Shibata Y; Makida Y; Fu GC Angew. Chem. Int. Ed 2017, 56, 5821. - PMC - PubMed
- Beak P; Basu A; Gallagher DJ; Park YS; Thayumanavan S Acc. Chem. Res 1996, 29, 552.
- Campos KR Chem. Soc. Rev 2007, 36, 1069. - PubMed
- Mitchell EA; Peschiulli A; Lefevre N; Meerpoel L; Maes BUW Chem. Eur. J 2012, 18, 10092. - PubMed
- Coldham I; Dufour S; Haxell TFN; Howard S; Vennall GP Angew. Chem. Int. Ed 2002, 41, 3887. - PubMed
- Beng TK; Gawley RE J. Am. Chem. Soc 2010, 132, 12216. - PMC - PubMed
- Millet A; Dailler D; Larini P; Baudoin O Angew. Chem. Int. Ed 2014, 53, 2678. - PubMed
- Watson RT; Gore VK; Chandupatla KR; Dieter RK; Snyder JP J. Org. Chem 2004, 69, 6105. - PubMed
- Klapars A; Campos KR; Waldman JH; Zewge D; Dormer PG; Chen C-Y J. Org. Chem 2008, 73, 4986. - PubMed
- Barker G; McGrath JL; Klapars A; Stead D; Zhou G; Campos KR; O’Brien P J. Org. Chem 2011, 76, 5936. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
