Age-related type I collagen modifications reveal tissue-defining differences between ligament and tendon
- PMID: 34825162
- PMCID: PMC8605237
- DOI: 10.1016/j.mbplus.2021.100070
Age-related type I collagen modifications reveal tissue-defining differences between ligament and tendon
Abstract
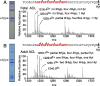
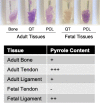
Tendons and ligaments tend to be pooled into a single category as dense elastic bands of collagenous connective tissue. They do have many similar properties, for example both tissues are flexible cords of fibrous tissue that join bone to either muscle or bone. Tendons and ligaments are both prone to degenerate and rupture with only limited capacity to heal, although tendons tend to heal faster than ligaments. Type I collagen constitutes about 80% of the dry weight of tendons and ligaments and is principally responsible for the core strength of each tissue. Collagen synthesis is a complex process with multiple steps and numerous post-translational modifications including proline and lysine hydroxylation, hydroxylysine glycosylation and covalent cross-linking. The chemistry, placement and quantity of intramolecular and intermolecular cross-links are believed to be key contributors to the tissue-specific variations in material strength and biological properties of collagens. As tendons and ligaments grow and develop, the collagen cross-links are known to chemically mature, strengthen and change in profile. Accordingly, changes in cross-linking and other post-translational modifications are likely associated with tissue development and degeneration. Using mass spectrometry, we have compared tendon and ligaments from fetal and adult bovine knee joints to investigate changes in collagen post-translational properties. Although hydroxylation levels at the type I collagen helical cross-linking lysine residues were similar in all adult tissues, ligaments had significantly higher levels of glycosylation at these sites compared to tendon. Differences in lysine hydroxylation were also found between the tissues at the telopeptide cross-linking sites. Total collagen cross-linking analysis, including mature trivalent cross-links and immature divalent cross-links, revealed unique cross-linking profiles between tendon and ligament tissues. Tendons were found to have a significantly higher frequency of smaller diameter collagen fibrils compared with ligament, which we suspect is functionally associated with the unique cross-linking profile of each tissue. Understanding the specific molecular characteristics that define and distinguish these specialized tissues will be important to improving the design of orthopedic treatment approaches.
Keywords: ACL, Anterior cruciate ligament; Collagen; Cross-linking; DHLNL, dehydrohydroxylysinonorleucine; HHL, histidinohydroxylysinonorleucine; HHMD, histidinohydroxymerodesmosine; HLNL, hydroxylysinonorleucine; HP, hydroxylysine pyridinoline; LC, liquid chromatography; LCL, lateral collateral ligament; LP, lysine pyridinoline; Ligament; MCL, medial collateral ligament; MS, mass spectrometry; Mass spectrometry; P3H1, prolyl 3-hydroxylase 1; P3H2, prolyl 3-hydroxylase 2; PCL, posterior cruciate ligament; Post-translational modifications; QT, quadriceps tendon; Tendon.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Distinct post-translational features of type I collagen are conserved in mouse and human periodontal ligament.J Periodontal Res. 2017 Dec;52(6):1042-1049. doi: 10.1111/jre.12475. Epub 2017 Jun 20. J Periodontal Res. 2017. PMID: 28631261 Free PMC article.
-
Cyclophilin-B Modulates Collagen Cross-linking by Differentially Affecting Lysine Hydroxylation in the Helical and Telopeptidyl Domains of Tendon Type I Collagen.J Biol Chem. 2016 Apr 29;291(18):9501-12. doi: 10.1074/jbc.M115.699470. Epub 2016 Mar 2. J Biol Chem. 2016. PMID: 26934917 Free PMC article.
-
Distinctive collagen maturation process in fibroblasts derived from rabbit anterior cruciate ligament, medial collateral ligament, and patellar tendon in vitro.Knee Surg Sports Traumatol Arthrosc. 2015 May;23(5):1384-1392. doi: 10.1007/s00167-013-2773-8. Epub 2013 Nov 13. Knee Surg Sports Traumatol Arthrosc. 2015. PMID: 24221246 Free PMC article.
-
Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance.Bone. 1998 Mar;22(3):181-7. doi: 10.1016/s8756-3282(97)00279-2. Bone. 1998. PMID: 9514209 Review.
-
Multiscale mechanical effects of native collagen cross-linking in tendon.Connect Tissue Res. 2018 Sep;59(5):410-422. doi: 10.1080/03008207.2018.1449837. Epub 2018 Jun 6. Connect Tissue Res. 2018. PMID: 29873266 Review.
Cited by
-
Endoscopic retrograde cholangiopancreatography induced splenic injury: comprehensive analysis and new perspectives based on a case report.Ther Adv Gastrointest Endosc. 2024 Jan 12;17:26317745231223312. doi: 10.1177/26317745231223312. eCollection 2024 Jan-Dec. Ther Adv Gastrointest Endosc. 2024. PMID: 38223215 Free PMC article.
-
Multiscale Characterization of Type I Collagen Fibril Stress-Strain Behavior under Tensile Load: Analytical vs. MD Approaches.Bioengineering (Basel). 2022 Apr 28;9(5):193. doi: 10.3390/bioengineering9050193. Bioengineering (Basel). 2022. PMID: 35621471 Free PMC article.
-
Temporal application of lysyl oxidase during hierarchical collagen fiber formation differentially effects tissue mechanics.Acta Biomater. 2023 Apr 1;160:98-111. doi: 10.1016/j.actbio.2023.02.024. Epub 2023 Feb 21. Acta Biomater. 2023. PMID: 36822485 Free PMC article.
-
Proteomic insights into the extracellular matrix: a focus on proteoforms and their implications in health and disease.Expert Rev Proteomics. 2024 Nov;21(11):463-481. doi: 10.1080/14789450.2024.2427136. Epub 2024 Nov 15. Expert Rev Proteomics. 2024. PMID: 39512072 Review.
-
Muscle impairments in osteogenesis imperfecta: a narrative review.JBMR Plus. 2025 Jun 5;9(8):ziaf099. doi: 10.1093/jbmrpl/ziaf099. eCollection 2025 Aug. JBMR Plus. 2025. PMID: 40642480 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

