Synthesis and antitumour evaluation of indole-2-carboxamides against paediatric brain cancer cells
- PMID: 34825187
- PMCID: PMC8597418
- DOI: 10.1039/d1md00065a
Synthesis and antitumour evaluation of indole-2-carboxamides against paediatric brain cancer cells
Abstract
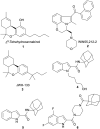
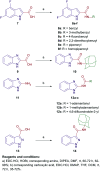
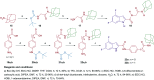
Paediatric glioblastomas are rapidly growing, devastating brain neoplasms with an invasive phenotype. Radiotherapy and chemotherapy, which are the current therapeutic adjuvant to surgical resection, are still associated with various toxicity profiles and only marginally improve the course of the disease and life expectancy. A considerable body of evidence supports the antitumour and apoptotic effects of certain cannabinoids, such as WIN55,212-2, against a wide spectrum of cancer cells, including gliomas. In fact, we previously highlighted the potent cytotoxic activity of the cannabinoid ligand 5 against glioblastoma KNS42 cells. Taken together, in this study, we designed, synthesised, and evaluated several indoles and indole bioisosteres for their antitumour activities. Compounds 8a, 8c, 8f, 12c, and 24d demonstrated significant inhibitory activities against the viability (IC50 = 2.34-9.06 μM) and proliferation (IC50 = 2.88-9.85 μM) of paediatric glioblastoma KNS42 cells. All five compounds further retained their antitumour activities against two atypical teratoid/rhabdoid tumour (AT/RT) cell lines. When tested against a medulloblastoma DAOY cell line, only 8c, 8f, 12c, and 24d maintained their viability inhibitory activities. The viability assay against non-neoplastic human fibroblast HFF1 cells suggested that compounds 8a, 8c, 8f, and 12c act selectively towards the panel of paediatric brain tumour cells. In contrast, compound 24d and WIN55,212-2 were highly toxic toward HFF1 cells. Due to their structural resemblance to known cannabimimetics, the most potent compounds were tested in cannabinoid 1 and 2 receptor (CB1R and CB2R) functional assays. Compounds 8a, 8c, and 12c failed to activate or antagonise both CB1R and CB2R, whereas compounds 8f and 24d antagonised CB1R and CB2R, respectively. We also performed a transcriptional analysis on KNS42 cells treated with our prototype compound 8a and highlighted a set of seven genes that were significantly downregulated. The expression levels of these genes were previously shown to be positively correlated with tumour growth and progression, indicating their implication in the antitumour activity of 8a. Overall, the drug-like and selective antitumour profiles of indole-2-carboxamides 8a, 8c, 8f, and 12c substantiate the versatility of the indole scaffold in cancer drug discovery.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Miklja Z. Pasternak A. Stallard S. Nicolaides T. Kline-Nunnally C. Cole B. Beroukhim R. Bandopadhayay P. Chi S. Ramkissoon S. H. Mullan B. Bruzek A. K. Gauthier A. Garcia T. Atchison C. Marini B. Fouladi M. Parsons D. W. Leary S. Mueller S. Ligon K. L. Koschmann C. Neuro-Oncology. 2019;21(8):968–980. doi: 10.1093/neuonc/noz022. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Chemical Information

