Combinatorial model of amyloid β and tau reveals synergy between amyloid deposits and tangle formation
- PMID: 34825397
- PMCID: PMC8810717
- DOI: 10.1111/nan.12779
Combinatorial model of amyloid β and tau reveals synergy between amyloid deposits and tangle formation
Abstract
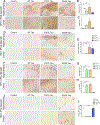
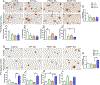
Aims: To illuminate the pathological synergy between Aβ and tau leading to emergence of neurofibrillary tangles (NFT) in Alzheimer's disease (AD), here, we have performed a comparative neuropathological study utilising three distinctive variants of human tau (WT tau, P301L mutant tau and S320F mutant tau). Previously, in non-transgenic mice, we showed that WT tau or P301L tau does not form NFT while S320F tau can spontaneously aggregate into NFT, allowing us to test the selective vulnerability of these different tau conformations to the presence of Aβ plaques.
Methods: We injected recombinant AAV-tau constructs into neonatal APP transgenic TgCRND8 mice or into 3-month-old TgCRND8 mice; both cohorts were aged 3 months post injection. This allowed us to test how different tau variants synergise with soluble forms of Aβ (pre-deposit cohort) or with frank Aβ deposits (post-deposit cohort).
Results: Expression of WT tau did not produce NFT or altered Aβ in either cohort. In the pre-deposit cohort, S320F tau induced Aβ plaque deposition, neuroinflammation and synaptic abnormalities, suggesting that early tau tangles affect the amyloid cascade. In the post-deposit cohort, contemporaneous expression of S320F tau did not exacerbate amyloid pathology, showing a dichotomy in Aβ-tau synergy based on the nature of Aβ. P301L tau produced NFT-type inclusions in the post-deposit cohort, but not in the pre-deposit cohort, indicating pathological synergy with pre-existing Aβ deposits.
Conclusions: Our data show that different tau mutations representing specific folding variants of tau synergise with Aβ to different extents, depending on the presence of cerebral deposits.
Keywords: Alzheimer's disease; gliosis; neurofibrillary tangle; neuroinflammation; pathological synergy; plaque burden.
© 2021 British Neuropathological Society.
Conflict of interest statement
Competing interests. The authors declare that they have no conflict of interest.
Figures






References
-
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–5. - PubMed
-
- Karran E, De Strooper B. The amyloid cascade hypothesis: are we poised for success or failure? Journal of neurochemistry. 2016;139 Suppl 2:237–52. - PubMed
-
- Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293(5534):1487–91. - PubMed
-
- Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293(5534):1491–5. - PubMed
-
- Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43(3):321–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

