Computational Study of Mechanism and Enantioselectivity of Imine Reductase from Amycolatopsis orientalis
- PMID: 34825518
- PMCID: PMC8734122
- DOI: 10.1002/open.202100250
Computational Study of Mechanism and Enantioselectivity of Imine Reductase from Amycolatopsis orientalis
Abstract
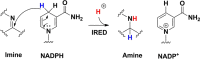
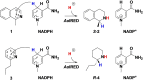
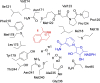
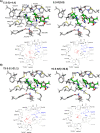
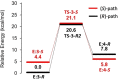
Imine reductases (IREDs) are NADPH-dependent enzymes (NADPH=nicotinamide adenine dinucleotide phosphate) that catalyze the reduction of imines to amines. They exhibit high enantioselectivity for a broad range of substrates, making them of interest for biocatalytic applications. In this work, we have employed density functional theory (DFT) calculations to elucidate the reaction mechanism and the origins of enantioselectivity of IRED from Amycolatopsis orientalis. Two substrates are considered, namely 1-methyl-3,4-dihydroisoquinoline and 2-propyl-piperideine. A model of the active site is built on the basis of the available crystal structure. For both substrates, different binding modes are first evaluated, followed by calculation of the hydride transfer transition states from each complex. We have also investigated the effect of mutations of certain important active site residues (Tyr179Ala and Asn241Ala) on the enantioselectivity. The calculated energies are consistent with the experimental observations and the analysis of transition states geometries provides insights into the origins of enantioselectivity of this enzyme.
Keywords: biocatalysis; enantioselectivity; imine reductase; reaction mechanism; transition state.
© 2021 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Schrittwieser J. H., Velikogne S., Kroutil W., Adv. Synth. Catal. 2015, 357, 1655.
-
- Ghislieri D., Turner N. J., Top. Catal. 2014, 57, 284.
-
- None
-
- Savile C. K., Janey J. M., Mundorff E. C., Moore J. C., Tam S., Jarvis W. R., Colbeck J. C., Krebber A., Fleitz F. J., Brands J., Devine P. N., Huisman G. W., Hughes G. J., Science 2010, 329, 305; - PubMed
-
- Busto E., Gotor-Fernandez V., Gotor V., Chem. Rev. 2011, 111, 3998; - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

