Heterogeneity of Tau Deposition and Microvascular Involvement in MCI and AD
- PMID: 34825871
- PMCID: PMC8822690
- DOI: 10.2174/1567205018666211126113904
Heterogeneity of Tau Deposition and Microvascular Involvement in MCI and AD
Abstract
Background: Reduced cerebrovascular function and accumulation of tau pathology are key components of cognitive decline in Alzheimer's disease (AD). Recent multimodal neuroimaging studies show a correlation between cortical tau accumulation and reduced cerebral perfusion. However, animal models predict that tau exerts capillary-level changes that may not be fully captured by standard imaging protocols.
Objective: Using newly-developed magnetic resonance imaging (MRI) technology to measure capillary- specific perfusion parameters, we examined a series of mild cognitive impairment (MCI) and AD patients with tau positron emission tomography (PET) to observe whole-brain capillary perfusion alterations and their association with tau deposition.
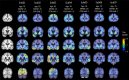
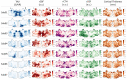
Methods: Seven subjects with MCI or AD received Flortaucipir PET to measure tau deposition and spin-echo dynamic susceptibility contrast (SE-DSC) MRI to measure microvascular perfusion (<10μm radius vessels). Gradient-echo (GE) DSC and pseudocontinuous arterial spin labeling (PCASL) MRI were also acquired to assess macrovascular perfusion. Tau PET, microvascular perfusion, and cortical thickness maps were visually inspected in volumetric slices and on cortical surface projections.
Results: High tau PET signal was generally observed in the lateral temporal and parietal cortices, with uptake in the occipital cortex in one subject. Global blood flow measured by PCASL was reduced with increasing tau burden, which was consistent with previous studies. Tau accumulation was spatially associated with variable patterns of microvascular cerebral blood flow (CBF) and oxygen extraction fraction (OEF) in the cortex and with increased capillary transit heterogeneity (CTH) in adjacent periventricular white matter, independent of amyloid-β status.
Conclusion: Although macrovascular perfusion generally correlated with tau deposition at the whole-cortex level, regional changes in microvascular perfusion were not uniformly associated with either tau pathology or cortical atrophy. This work highlights the heterogeneity of AD-related brain changes and the challenges of implementing therapeutic interventions to improve cerebrovascular function.
Keywords: Alzheimer's disease; cerebrovascular function.; dynamic susceptibility contrast magnetic resonance imaging; microvascular perfusion; multimodal neuroimaging; positron emission tomography; tau pathology.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Figures

References
-
- Mattsson-Carlgren N., Andersson E., Janelidze S., Ossenkoppele R., Insel P., Strandberg O., Zetterberg H., Rosen H.J., Rabinovici G., Chai X., Blennow K., Dage J.L., Stomrud E., Smith R., Palmqvist S., Hansson O. Aβ deposition is associated with increases in soluble and phosphorylated tau that precede a positive Tau PET in Alzheimer’s disease. Sci. Adv. 2020;6(16):eaaz2387. doi: 10.1126/sciadv.aaz2387. - DOI - PMC - PubMed
-
- Schöll M., Lockhart S.N., Schonhaut D.R., O’Neil J.P., Janabi M., Ossenkoppele R., Baker S.L., Vogel J.W., Faria J., Schwimmer H.D., Rabinovici G.D., Jagust W.J. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89(5):971–982. doi: 10.1016/j.neuron.2016.01.028. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

