Corticosteroid treatment and mortality in mechanically ventilated COVID-19-associated acute respiratory distress syndrome (ARDS) patients: a multicentre cohort study
- PMID: 34825976
- PMCID: PMC8617372
- DOI: 10.1186/s13613-021-00951-0
Corticosteroid treatment and mortality in mechanically ventilated COVID-19-associated acute respiratory distress syndrome (ARDS) patients: a multicentre cohort study
Abstract
Background: Some unanswered questions persist regarding the effectiveness of corticosteroids for severe coronavirus disease 2019 (COVID-19) patients. We aimed to assess the clinical effect of corticosteroids on intensive care unit (ICU) mortality among mechanically ventilated COVID-19-associated acute respiratory distress syndrome (ARDS) patients.
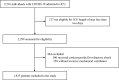
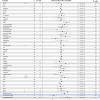
Methods: This was a retrospective study of prospectively collected data conducted in 70 ICUs (68 Spanish, one Andorran, one Irish), including mechanically ventilated COVID-19-associated ARDS patients admitted between February 6 and September 20, 2020. Individuals who received corticosteroids for refractory shock were excluded. Patients exposed to corticosteroids at admission were matched with patients without corticosteroids through propensity score matching. Primary outcome was all-cause ICU mortality. Secondary outcomes were to compare in-hospital mortality, ventilator-free days at 28 days, respiratory superinfection and length of stay between patients with corticosteroids and those without corticosteroids. We performed survival analysis accounting for competing risks and subgroup sensitivity analysis.
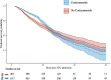
Results: We included 1835 mechanically ventilated COVID-19-associated ARDS, of whom 1117 (60.9%) received corticosteroids. After propensity score matching, ICU mortality did not differ between patients treated with corticosteroids and untreated patients (33.8% vs. 30.9%; p = 0.28). In survival analysis, corticosteroid treatment at ICU admission was associated with short-term survival benefit (HR 0.53; 95% CI 0.39-0.72), although beyond the 17th day of admission, this effect switched and there was an increased ICU mortality (long-term HR 1.68; 95% CI 1.16-2.45). The sensitivity analysis reinforced the results. Subgroups of age < 60 years, severe ARDS and corticosteroids plus tocilizumab could have greatest benefit from corticosteroids as short-term decreased ICU mortality without long-term negative effects were observed. Larger length of stay was observed with corticosteroids among non-survivors both in the ICU and in hospital. There were no significant differences for the remaining secondary outcomes.
Conclusions: Our results suggest that corticosteroid treatment for mechanically ventilated COVID-19-associated ARDS had a biphasic time-dependent effect on ICU mortality. Specific subgroups showed clear effect on improving survival with corticosteroid use. Therefore, further research is required to identify treatment-responsive subgroups among the mechanically ventilated COVID-19-associated ARDS patients.
Keywords: COVID-19-associated acute respiratory distress syndrome; Corticosteroids; Intensive care unit; Invasive mechanical ventilation; Mortality.
© 2021. The Author(s).
Conflict of interest statement
PV-C reports personal fees and non-financial support from Pfizer, personal fees and non-financial support from MSD, personal fees from Shionogi, personal fees from Menarini, outside the submitted work. RF reports personal fees from Jafron, personal fees from Toray, personal fees from ThermoFisher, personal fees from Grifols, personal fees from Pfizer, personal fees from MSD, personal fees from Becton-Dickinson, personal fees from Shionogi, outside the submitted work. All other authors declare no competing interests.
Figures

References
-
- World Health Organization. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int. Accessed 3 Mar 2021.
-
- Marmor M, Jonas A. Corticosteroids for COVID-19-associated ARDS. Clin Pulm Med. 2020;27:165–167.
Grants and funding
LinkOut - more resources
Full Text Sources

