In vitro evaluation of periapical lesion-derived stem cells for dental pulp tissue engineering
- PMID: 34826215
- PMCID: PMC8727956
- DOI: 10.1002/2211-5463.13336
In vitro evaluation of periapical lesion-derived stem cells for dental pulp tissue engineering
Abstract
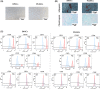
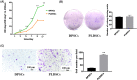
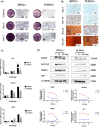
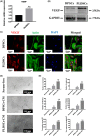
Dental pulp tissue engineering is a promising alternative treatment for pulpitis and periapical periodontitis, and dental pulp stem cells (DPSCs) are considered to be the gold standard for dental seed cell research. Periapical lesions harbor mesenchymal stem cells with the capacity for self-renewal and multilineage differentiation. However, it remains unknown whether these periapical lesion-derived stem cells (PLDSCs) are suitable for dental pulp tissue engineering. To investigate this possibility, PLDSCs and DPSCs were isolated using the tissue outgrowth method and cultured under identical conditions. We then performed in vitro experiments to investigate their biological characteristics. Our results indicate that PLDSCs proliferate actively in vitro and exhibit similar morphology, immunophenotype and multilineage differentiation ability as DPSCs. Simultaneously, PLDSCs exhibit stronger migrative ability and express more vascular endothelial growth factor and glial cell line-derived neurotrophic factor than DPSCs, and PLDSC-derived conditioned medium was more effective in tube formation assay. The mRNA expression levels of immunomodulatory genes HLA-G, IDO and ICAM-1 were also higher in PLDSCs. However, regarding osteo/odontogenic differentiation, PLDSCs showed weaker alkaline phosphatase staining and lower calcified nodule formation compared to DPSCs, as well as lower expression of ALP, RUNX2 and DSPP, as confirmed by a quantitative RT-PCR. The osteo/odontogenic protein expression levels of DSPP, RUNX2, DMP1 and SP7 were also higher in DPSCs. The present study demonstrates that PLDSCs demonstrate potential use as seed cells for dental pulp regeneration, especially for achieving enhanced neurovascularization.
Keywords: dental pulp regeneration; immunomodulatory; odontogenesis; osteogenesis; periapical lesion-derived stem cells; pro-angiogenesis.
© 2021 The Authors. FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
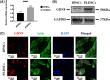
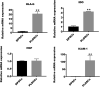
References
-
- Xuan K, Li B, Guo H, Sun W, Kou X, He X, et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med. 2018;10:455. - PubMed
-
- Gronthos S, Brahim J, Li W, Fisher LW, Cherman BA, DenBesten P, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–5. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

