ADAM17/MMP inhibition prevents neutrophilia and lung injury in a mouse model of COVID-19
- PMID: 34826347
- PMCID: PMC9015574
- DOI: 10.1002/JLB.3COVA0421-195RR
ADAM17/MMP inhibition prevents neutrophilia and lung injury in a mouse model of COVID-19
Abstract
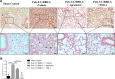
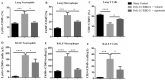
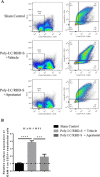
Severe coronavirus disease 2019 (COVID-19) is characterized by lung injury, cytokine storm, and increased neutrophil-to-lymphocyte ratio (NLR). Current therapies focus on reducing viral replication and inflammatory responses, but no specific treatment exists to prevent the development of severe COVID-19 in infected individuals. Angiotensin-converting enzyme-2 (ACE2) is the receptor for SARS-CoV-2, the virus causing COVID-19, but it is also critical for maintaining the correct functionality of lung epithelium and endothelium. Coronaviruses induce activation of a disintegrin and metalloprotease 17 (ADAM17) and shedding of ACE2 from the cell surface resulting in exacerbated inflammatory responses. Thus, we hypothesized that ADAM17 inhibition ameliorates COVID-19-related lung inflammation. We employed a preclinical mouse model using intratracheal instillation of a combination of polyinosinic:polycytidylic acid (poly(I:C)) and the receptor-binding domain of the SARS-CoV-2 spike protein (RBD-S) to mimic lung damage associated with COVID-19. Histologic analysis of inflamed mice confirmed the expected signs of lung injury including edema, fibrosis, vascular congestion, and leukocyte infiltration. Moreover, inflamed mice also showed an increased NLR as observed in critically ill COVID-19 patients. Administration of the ADAM17/MMP inhibitors apratastat and TMI-1 significantly improved lung histology and prevented leukocyte infiltration. Reduced leukocyte recruitment could be explained by reduced production of proinflammatory cytokines and lower levels of the endothelial adhesion molecules ICAM-1 and VCAM-1. Additionally, the NLR was significantly reduced by ADAM17/MMP inhibition. Thus, we propose inhibition of ADAM17/MMP as a novel promising treatment strategy in SARS-CoV-2-infected individuals to prevent the progression toward severe COVID-19.
Keywords: IL-10; MMP; TACE; TNF-α; acute lung injury; endothelium; lymphocyte; neutrophil.
©2021 Society for Leukocyte Biology.
Figures


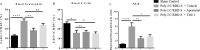

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

