Physical therapy exerts sub-additive and suppressive effects on intracerebral neural stem cell implantation in a rat model of stroke
- PMID: 34826373
- PMCID: PMC9254031
- DOI: 10.1177/0271678X211062955
Physical therapy exerts sub-additive and suppressive effects on intracerebral neural stem cell implantation in a rat model of stroke
Abstract
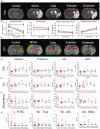
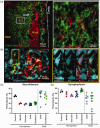
Intracerebral cell therapy (CT) is emerging as a new therapeutic paradigm for stroke. However, the impact of physical therapy (PT) on implanted cells and their ability to promote recovery remains poorly understood. To address this translational issue, a clinical-grade neural stem cell (NSC) line was implanted into peri-infarct tissue using MRI-defined injection sites, two weeks after stroke. PT in the form of aerobic exercise (AE) was administered 5 × per week post-implantation using a paradigm commonly applied in patients with stroke. A combined AE and CT exerted sub-additive therapeutic effects on sensory neglect, whereas AE suppressed CT effects on motor integration and grip strength. Behavioral testing emerged as a potentially major component for task integration. It is expected that this study will guide and inform the incorporation of PT in the design of clinical trials evaluating intraparenchymal NSCs implantation for stroke.
Keywords: Physical therapy; aerobic exercise; cell therapy; neural stem cell; neurorehabilitation; stroke.
Conflict of interest statement
Figures





References
-
- Boltze J, Modo MM, Mays RW, et al.. Stem cells as an emerging paradigm in stroke 4: Advancing and accelerating preclinical research. Stroke 2019; 50: 3299–3306. - PubMed
-
- Smith EJ, Stroemer RP, Gorenkova N, et al.. Implantation site and lesion topology determine efficacy of a human neural stem cell line in a rat model of chronic stroke. Stem Cells 2012; 30: 785–796. - PubMed
-
- Stroemer P, Patel S, Hope A, et al.. The neural stem cell line CTX0E03 promotes behavioral recovery and endogenous neurogenesis after experimental stroke in a dose-dependent fashion. Neurorehabil Neural Repair 2009; 23: 895–909. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

