SARS-CoV-2 RT-qPCR Test Detection Rates Are Associated with Patient Age, Sex, and Time since Diagnosis
- PMID: 34826637
- PMCID: PMC8608683
- DOI: 10.1016/j.jmoldx.2021.10.010
SARS-CoV-2 RT-qPCR Test Detection Rates Are Associated with Patient Age, Sex, and Time since Diagnosis
Abstract
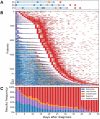
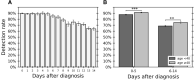
Quantifying the detection rate of the widely used quantitative RT-PCR (RT-qPCR) test for severe acute respiratory syndrome coronavirus 2 and its dependence on patient demographic characteristics and disease progression is key in designing epidemiologic strategies. Analyzing 843,917 test results of 521,696 patients, a "positive period" was defined for each patient between diagnosis of coronavirus disease 2019 and the last positive test result. The fraction of positive test results within this period was then used to estimate detection rate. Regression analyses were used to determine associations of detection with time of sampling after diagnosis, patient demographic characteristics, and viral RNA copy number based on RT-qPCR cycle threshold values of the next positive test result. The overall detection rate in tests performed within 14 days after diagnosis was 83.1%. This rate was higher at days 0 to 5 after diagnosis (89.3%). Furthermore, detection rate was strongly associated with age and sex. Finally, the detection rate with the Allplex 2019-nCoV RT-qPCR kit was associated, at the single-patient level, with viral RNA copy number (P < 10-9). These results show that the reliability of the test result is reduced in later days as well as for women and younger patients, in whom the viral loads are typically lower.
Copyright © 2022 Association for Molecular Pathology and American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Bisoffi Z., Pomari E., Deiana M., Piubelli C., Ronzoni N., Beltrame A., Bertoli G., Riccardi N., Perandin F., Formenti F., Gobbi F., Buonfrate D., Silva R. Sensitivity, specificity and predictive values of molecular and serological tests for COVID-19: a longitudinal study in emergency room. Diagnostics (Basel) 2020;10:669. - PMC - PubMed
-
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

