Short-Term Cardiovascular Effects of E-Cigarettes in Adults Making a Stop-Smoking Attempt: A Randomized Controlled Trial
- PMID: 34827200
- PMCID: PMC8614829
- DOI: 10.3390/biology10111208
Short-Term Cardiovascular Effects of E-Cigarettes in Adults Making a Stop-Smoking Attempt: A Randomized Controlled Trial
Abstract
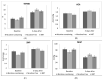
Smoking increases cardiovascular disease (CVD) risk by leading to endothelial injury. E-cigarettes remain a popular way to stop smoking. Evidence on their effect on cardiovascular health is growing but remains limited, particularly in the short-term. The main objective of this study was to compare short-term cardiovascular effects in smokers who quit smoking using e-cigarettes with or without nicotine or prescription nicotine replacement therapy (NRT). This was a single-centre (Sheffield, UK) pragmatic three-arm randomised controlled trial which recruited adult smokers (≥10 cigarettes per day), who were willing to attempt to stop smoking with support (n = 248). Participants were randomised to receive either: (a) behavioral support and e-cigarettes with 18 mg/mL nicotine (n = 84); (b) behavioral support and e-cigarettes without nicotine (n = 82); (c) behavioral support and NRT (n = 82). Flow Mediated Dilation (%FMD), peak cutaneous vascular conductance responses to acetylcholine (ACh) and sodium nitroprusside (SNP) and mean arterial pressure (MAP) were recorded at baseline and three days after stopping smoking. General Linear Models were used to compare changes between groups and changes from follow-up. Adjusting for baseline, at follow-up, all outcomes (for the 208 participants that completed the 3-day assessments) with the exception of SNP had improved significantly over baseline and there were no differences between groups (%FMD F = 1.03, p = 0.360, df = 2,207; ACh F = 0.172, p = 0.84, df = 2,207; SNP F = 0.382, p = 0.68, df = 2,207; MAP F = 0.176, p = 0.84, df = 2,207). For smokers ≥20 cigarettes per day, benefits were also pronounced. Smoking cessation showed positive cardiovascular impact even after a 3-day period and the effects did not differ between nicotine-containing e-cigarettes, nicotine-free e-cigarettes and NRT.
Keywords: CVD; nicotine replacement therapy; smoking cessation; vaping; vascular function.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Centers for Disease Control and Prevention (US), National Center for Chronic Disease Prevention and Health Promotion (US) & Office on Smoking and Health (US) How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Centers for Disease Control and Prevention (US); Atlanta, GA, USA: 2010. - PubMed
-
- Office on Smoking and Health (US) The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Centers for Disease Control and Prevention (US); Atlanta, GA, USA: 2006. - PubMed
-
- U.S. Department of Health & Human Services. Smoking Cessation: A Report of the Surgeon General. Centers for Disease Control and Prevention; Atlanta, GA, USA: 2020.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

