Molnupiravir-A Novel Oral Anti-SARS-CoV-2 Agent
- PMID: 34827232
- PMCID: PMC8614993
- DOI: 10.3390/antibiotics10111294
Molnupiravir-A Novel Oral Anti-SARS-CoV-2 Agent
Abstract
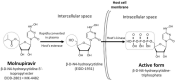
Since December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly resulted in a global pandemic with approximately 4 million deaths. Effective oral antiviral agents are urgently needed to treat coronavirus disease-2019 (COVID-19), block SARS-CoV-2 transmission, and prevent progression to severe illness. Molnupiravir (formerly EIDD-2801), a prodrug of beta-d-N4-hydroxycytidine (EIDD-1931) and an inhibitor of RNA-dependent RNA polymerase, possesses significant activity against SARS-CoV-2. Its prophylactic efficacy has been evidenced in a ferret model. Two phase-I trials (NCT04392219 and NCT04746183) have demonstrated that oral molnupiravir is safe and well-tolerated at therapeutic doses. After five-days of oral molnupiravir therapy, satisfactory efficacies, assessed by eliminating nasopharyngeal virus in patients with early and mild COVID-19, were disclosed in two phase-II trials (NCT04405739 and NCT04405570). Two phase-II/III trials, NCT04575597 and NCT04575584, with estimated enrollments of 1850 and 304 cases, respectively, are ongoing. The NCT04575597 recently released that molnupiravir significantly reduced the risk of hospitalization or death in adults experiencing mild or moderate COVID-19. To benefit individual and public health, clinical applications of molnupiravir to promptly treat COVID-19 patients and prevent SARS-CoV-2 transmission may be expected.
Keywords: COVID-19; RNA polymerase inhibitor; antiviral agents; clinical trial; molnupiravir.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous