A Culture Change: Impact of a Pediatric Antimicrobial Stewardship Program Based on Guideline Implementation and Prospective Audit with Feedback
- PMID: 34827245
- PMCID: PMC8614734
- DOI: 10.3390/antibiotics10111307
A Culture Change: Impact of a Pediatric Antimicrobial Stewardship Program Based on Guideline Implementation and Prospective Audit with Feedback
Abstract
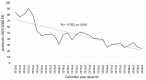
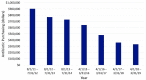
Reports analyzing the impact of pediatric antimicrobial stewardship programs (ASP) over long periods of time are lacking. We thus report our ASP experience in a pediatric tertiary referral center over a long-term period from 2011 to 2018. Our ASP was implemented in 2011. The program was based primarily on guideline development with key stakeholders, engaging and educating providers, followed by prospective audit with feedback (PAF). Monitored antibiotics included meropenem, piperacillin-tazobactam, and cefepime, followed by the addition of ceftriaxone, ceftazidime, cefotaxime, ciprofloxacin, levofloxacin, linezolid, and vancomycin at various time points. Specifically, the program did not implemented the core strategy of formulary restriction with prior authorization. Process- and outcome-related ASP measures were analyzed. We saw a 32% decrease in overall antibiotic utilization, a 51% decrease in the utilization of antibiotics undergoing PAF, and a 72% reduction in the use of broad-spectrum antibiotics such as meropenem. There was a concomitant increase in organism susceptibility and a reduction in yearly drug purchasing costs of over USD 560,000 from baseline without changes in sepsis-related mortality. Our study highlights that a pediatric ASP based primarily on the principles of guideline development and PAF can improve antibiotic utilization and institutional bacterial susceptibilities without a detrimental impact on patient outcomes by changing the culture of antimicrobial utilization within the institution.
Keywords: antibiotics; antimicrobial resistance; antimicrobial stewardship; pediatric; sepsis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bronzwaer S.L., Cars O., Buchholz U., Molstad S., Goettsch W., Veldhuijzen I.K., Kool J.L., Sprenger M.J., Degener J.E., European Antimicrobial Resistance Surveillance System A European study on the relationship between antimicrobial use and antimicrobial resistance. Emerg. Infect. Dis. 2002;8:278–282. doi: 10.3201/eid0803.010192. - DOI - PMC - PubMed
-
- United Nations Political Declaration of the High-Level Meeting of the General Assembly on Antimicrobial Resistance. 2016. [(accessed on 1 October 2021)]. Available online: https://digitallibrary.un.org/record/842813?ln=en.
LinkOut - more resources
Full Text Sources

