A Retrospective Analysis of Randomized Controlled Trials on Traumatic Brain Injury: Evaluation of CONSORT Item Adherence
- PMID: 34827503
- PMCID: PMC8615648
- DOI: 10.3390/brainsci11111504
A Retrospective Analysis of Randomized Controlled Trials on Traumatic Brain Injury: Evaluation of CONSORT Item Adherence
Abstract
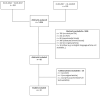
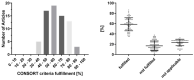
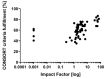
Traumatic brain injury (TBI) contributes to death and disability, resulting in an enormous individual and socio-economic challenges. Despite huge efforts, there are still controversies on treatment strategies and early outcome estimation. We evaluate current randomized controlled trials (RCTs) on TBI according to their fulfillment of the CONSORT (Consolidated Statement of Reporting Trials) statement's criteria as a marker of transparency and the quality of study planning and realization. A PubMed search for RCTs on TBI (January 2014-December 2019) was carried out. After screening of the abstracts (n = 1.926), the suitable full text manuscripts (n = 72) were assessed for the fulfillment of the CONSORT criteria. The mean ratio of consort statement fulfillment was 59% (±13%), 31% of the included studies (n = 22) complied with less than 50% of the CONSORT criteria. Citation frequency was moderately related to ratio of CONSORT item fulfillment (r = 0.4877; p < 0.0001) and citation frequency per year (r = 0.5249; p < 0.0001). The ratio of CONSORT criteria fulfillment was associated with the impact factor of the publishing journal (r = 0.6428; p < 0.0001). Essential data for study interpretation, such as sample size determination (item 7a), participant flow (item 13a) as well as losses and exclusions (item 13b), were only reported in 53%, 60% and 63%, respectively. Reporting and methodological aspects in RCTs on TBI still may be improved. Thus, the interpretation of study results may be hampered due to methodological weaknesses.
Keywords: CONSORT criteria; TBI; methodology; randomized controlled trial.
Conflict of interest statement
No conflict of interest to declare.
Figures

Similar articles
-
Randomized Controlled Trials on Intracerebral Hemorrhage: A Cross Sectional Retrospective Analysis of CONSORT Item Adherence.Front Neurol. 2019 Sep 20;10:991. doi: 10.3389/fneur.2019.00991. eCollection 2019. Front Neurol. 2019. PMID: 31616358 Free PMC article.
-
Randomized controlled trials in adult traumatic brain injury: a review of compliance to CONSORT statement.Arch Phys Med Rehabil. 2015 Apr;96(4):702-14. doi: 10.1016/j.apmr.2014.10.026. Epub 2014 Dec 9. Arch Phys Med Rehabil. 2015. PMID: 25497515 Review.
-
Analysis of the quality of reporting of randomized controlled trials in acute and chronic myeloid leukemia, and myelodysplastic syndromes as governed by the CONSORT statement.Ann Epidemiol. 2009 Jul;19(7):494-500. doi: 10.1016/j.annepidem.2009.03.018. Ann Epidemiol. 2009. PMID: 19523596
-
CONSORT item adherence in top ranked anaesthesiology journals in 2011: a retrospective analysis.Eur J Anaesthesiol. 2015 Feb;32(2):117-25. doi: 10.1097/EJA.0000000000000176. Eur J Anaesthesiol. 2015. PMID: 25387297
-
Assessment of the reporting quality of RCTs for novel oral anticoagulants in venous thromboembolic disease based on the CONSORT statement.J Thromb Thrombolysis. 2019 Nov;48(4):542-553. doi: 10.1007/s11239-019-01931-9. J Thromb Thrombolysis. 2019. PMID: 31401718 Review.
Cited by
-
Assessment of the reporting quality of randomised controlled trials for vitamin D supplementation in autoimmune thyroid disorders based on the CONSORT statement.Endocrine. 2023 May;80(2):346-354. doi: 10.1007/s12020-022-03270-x. Epub 2022 Dec 3. Endocrine. 2023. PMID: 36462148 Free PMC article.
References
-
- James S.L., Theadom A., Ellenbogen R.G., Bannick M.S., Montjoy-Venning W., Lucchesi L.R., Abbasi N., Abdulkader R., Abraha H.N., Adsuar J.C., et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;18:56–87. doi: 10.1016/S1474-4422(18)30415-0. - DOI - PMC - PubMed
-
- Zaloshnja E., Miller T., Langlois J.A., Selassie A.W. Prevalence of Long-Term Disability from Traumatic Brain Injury in the Civilian Population of the United States, 2005. J. Head Trauma Rehabil. 2008;23:394–400. doi: 10.1097/01.HTR.0000341435.52004.ac. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

