Antioxidant and Anti-Inflammatory Effects of 3-Dehydroxyceanothetric Acid 2-Methyl Ester Isolated from Ziziphus jujuba Mill. against Cisplatin-Induced Kidney Epithelial Cell Death
- PMID: 34827612
- PMCID: PMC8615384
- DOI: 10.3390/biom11111614
Antioxidant and Anti-Inflammatory Effects of 3-Dehydroxyceanothetric Acid 2-Methyl Ester Isolated from Ziziphus jujuba Mill. against Cisplatin-Induced Kidney Epithelial Cell Death
Abstract
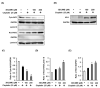
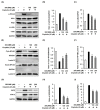
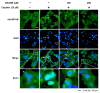
Cisplatin is a platinum-based chemotherapeutic agent for treating solid tumors; however, it presents a risk factor for nephropathy. In the present study, we investigated the antioxidant and anti-inflammatory effects of 3-dehydroxyceanothetric acid 2-methyl ester (3DC2ME) isolated from Ziziphus jujuba Mill. in LLC-PK1 cells following cisplatin-induced cytotoxicity. These cells were exposed to 3DC2ME for 2 h, followed by treatment with cisplatin for 24 h. The treated cells were subjected to cell viability analysis using the Ez-Cytox assay. Reactive oxygen species (ROS) were detected via 2', 7'- dichlorodihydrofluorescein diacetate (DCFH-DA) staining. In addition, western blotting and fluorescent immunostaining were performed to evaluate protein expressions related to oxidative stress and inflammation pathways. Pretreatment with 3DC2ME protected LLC-PK1 cells from cisplatin-induced cytotoxicity and oxidative stress. In addition, pretreatment with 3DC2ME upregulated heme oxygenase 1 (HO-1) via the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway in the cisplatin-treated LLC-PK1 cells. Furthermore, the increase in the expressions of IκB kinase α/β (IKKα/β), inhibitor of kappa B alpha (IκBα), nuclear factor kappa B (NF-κB), inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) in these cells was inhibited. These results provide basic scientific evidence for understanding the antioxidant and anti-inflammatory effects of 3DC2ME isolated from Z. jujuba against cisplatin-induced kidney epithelial cell death.
Keywords: LLC-PK1; Ziziphus jujuba Mill.; inflammation; nephrotoxicity; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

