Expanding the Disorder-Function Paradigm in the C-Terminal Tails of Erbbs
- PMID: 34827688
- PMCID: PMC8615588
- DOI: 10.3390/biom11111690
Expanding the Disorder-Function Paradigm in the C-Terminal Tails of Erbbs
Abstract
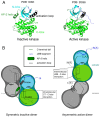
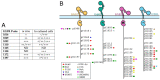
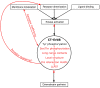
ErbBs are receptor tyrosine kinases involved not only in development, but also in a wide variety of diseases, particularly cancer. Their extracellular, transmembrane, juxtamembrane, and kinase folded domains were described extensively over the past 20 years, structurally and functionally. However, their whole C-terminal tails (CTs) following the kinase domain were only described at atomic resolution in the last 4 years. They were shown to be intrinsically disordered. The CTs are known to be tyrosine-phosphorylated when the activated homo- or hetero-dimers of ErbBs are formed. Their phosphorylation triggers interaction with phosphotyrosine binding (PTB) or Src Homology 2 (SH2) domains and activates several signaling pathways controling cellular motility, proliferation, adhesion, and apoptosis. Beyond this passive role of phosphorylated domain and site display for partners, recent structural and function studies unveiled active roles in regulation of phosphorylation and interaction: the CT regulates activity of the kinase domain; different phosphorylation states have different compaction levels, potentially modulating the succession of phosphorylation events; and prolines have an important role in structure, dynamics, and possibly regulatory interactions. Here, we review both the canonical role of the disordered CT domains of ErbBs as phosphotyrosine display domains and the recent findings that expand the known range of their regulation functions linked to specific structural and dynamic features.
Keywords: EGFR; ErbB; HER; intrinsic disorder; receptor tyrosine kinases; signal transduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

