Functions and Inhibition of Galectin-7, an Emerging Target in Cellular Pathophysiology
- PMID: 34827718
- PMCID: PMC8615947
- DOI: 10.3390/biom11111720
Functions and Inhibition of Galectin-7, an Emerging Target in Cellular Pathophysiology
Abstract
Galectin-7 is a soluble unglycosylated lectin that is able to bind specifically to β-galactosides. It has been described to be involved in apoptosis, proliferation and differentiation, but also in cell adhesion and migration. Several disorders and diseases are discussed by covering the aforementioned biological processes. Structural features of galectin-7 are discussed as well as targeting the protein intracellularly or extracellularly. The exact molecular mechanisms that lie behind many biological processes involving galectin-7 are not known. It is therefore useful to come up with chemical probes or tools in order to obtain knowledge of the physiological processes. The objective of this review is to summarize the roles and functions of galectin-7 in the human body, providing reasons why it is necessary to design inhibitors for galectin-7, to give the reader structural insights and describe its current inhibitors.
Keywords: apoptosis; epithelial tissues; galectin-7; inhibitors; targeting.
Conflict of interest statement
The author declares no conflict of interest.
Figures

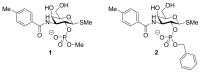



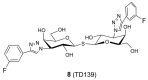
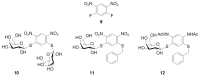
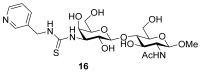

References
-
- Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., Darvill A.G., Kinoshita T., Packer N.H., Prestegard J.H., et al. Essentials of Glycobiology. 3rd ed. Cold Spring Harbor Laboratory Press; New York, NY, USA: 2017. - PubMed
-
- Si Y., Yao Y., Jaramillo Ayala G., Li X., Han Q., Zhang W., Xu X., Tai G., Mayo K.H., Zhou Y., et al. Human galectin-16 has a pseudo ligand binding site and plays a role in regulating c-Rel-mediated lymphocyte activity. Biochim. Biophys. Acta Gen. Subj. 2021;1865:129755. doi: 10.1016/j.bbagen.2020.129755. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

