β-Dystroglycan Restoration and Pathology Progression in the Dystrophic mdx Mouse: Outcome and Implication of a Clinically Oriented Study with a Novel Oral Dasatinib Formulation
- PMID: 34827740
- PMCID: PMC8615430
- DOI: 10.3390/biom11111742
β-Dystroglycan Restoration and Pathology Progression in the Dystrophic mdx Mouse: Outcome and Implication of a Clinically Oriented Study with a Novel Oral Dasatinib Formulation
Abstract
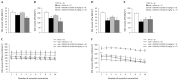
ROS-activated cSrc tyrosine kinase (TK) promotes the degradation of β-dystroglycan (β-DG), a dystrophin-glycoprotein complex component, which may reinforce damaging signals in Duchenne muscular dystrophy (DMD). Therefore, cSrc-TK represents a promising therapeutic target. In mdx mice, a 4-week subcutaneous treatment with dasatinib (DAS), a pan-Src-TKs inhibitor approved as anti-leukemic agent, increased muscle β-DG, with minimal amelioration of morphofunctional indices. To address possible dose/pharmacokinetic (PK) issues, a new oral DAS/hydroxypropyl(HP)-β-cyclodextrin(CD) complex was developed and chronically administered to mdx mice. The aim was to better assess the role of β-DG in pathology progression, meanwhile confirming DAS mechanism of action over the long-term, along with its efficacy and tolerability. The 4-week old mdx mice underwent a 12-week treatment with DAS/HP-β-CD10% dissolved in drinking water, at 10 or 20 mg/kg/day. The outcome was evaluated via in vivo/ex vivo disease-relevant readouts. Oral DAS/HP-β-CD efficiently distributed in mdx mice plasma and tissues in a dose-related fashion. The new DAS formulation confirmed its main upstream mechanism of action, by reducing β-DG phosphorylation and restoring its levels dose-dependently in both diaphragm and gastrocnemius muscle. However, it modestly improved in vivo neuromuscular function, ex vivo muscle force, and histopathology, although the partial recovery of muscle elasticity and the decrease of CK and LDH plasma levels suggest an increased sarcolemmal stability of dystrophic muscles. Our clinically oriented study supports the interest in this new, pediatric-suitable DAS formulation for proper exposure and safety and for enhancing β-DG expression. This latter mechanism is, however, not sufficient by itself to impact on pathology progression. In-depth analyses will be dedicated to elucidating the mechanism limiting DAS effectiveness in dystrophic settings, meanwhile assessing its potential synergy with dystrophin-based molecular therapies.
Keywords: Duchenne muscular dystrophy; cyclodextrin; dasatinib; mdx mouse; oral formulation; pediatric age; preclinical study.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
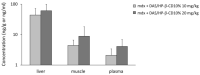


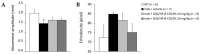


References
-
- Birnkrant D.J., Bushby K., Bann C.M., Apkon S.D., Blackwell A., Brumbaugh D., Case L.E., Clemens P.R., Hadjiyannakis S., Pandya S., et al. DMD Care Considerations Working Group. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018;17:251–267. doi: 10.1016/S1474-4422(18)30024-3. - DOI - PMC - PubMed
-
- Mantuano P., Sanarica F., Conte E., Morgese M.G., Capogrosso R.F., Cozzoli A., Fonzino A., Quaranta A., Rolland J.F., De Bellis M., et al. Effect of a long-term treatment with metformin in dystrophic mdx mice: A reconsideration of its potential clinical interest in Duchenne muscular dystrophy. Biochem. Pharmacol. 2018;154:89–103. doi: 10.1016/j.bcp.2018.04.022. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

